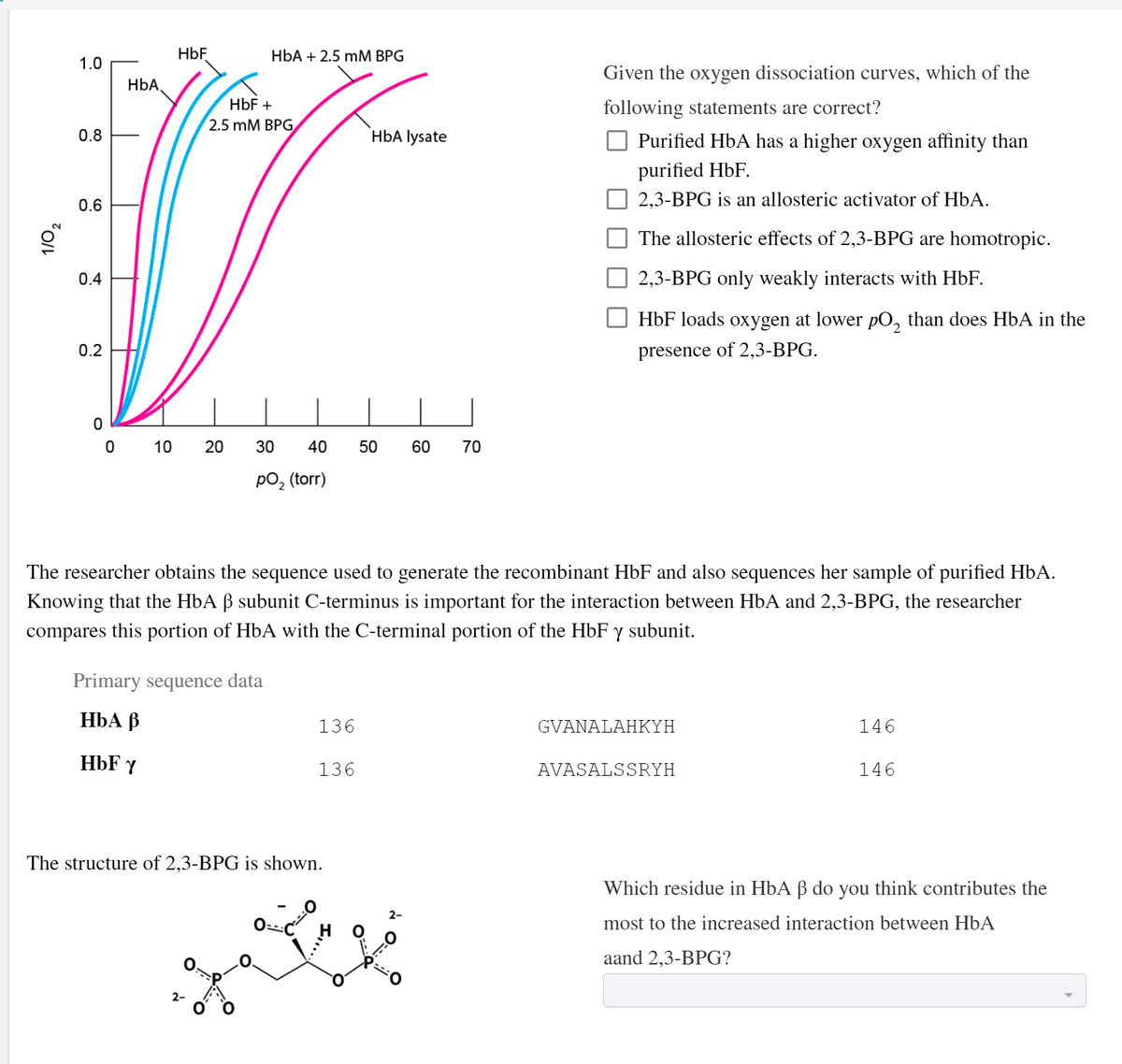
HbF HbA + 2.5 mM BPG 1.0 Given the oxygen dissociation curves, which of the HbA, HbF + following statements are correct? 2.5 mM BPG 0.8 HbA lysate O Purified HbA has a higher oxygen affinity than purified HbF. 0.6 2,3-BPG is an allosteric activator of HbA. The allosteric effects of 2,3-BPG are homotropic. 0.4 2,3-BPG only weakly interacts with HbF. HbF loads oxygen at lower pO, than does HbA in the 0.2 presence of 2,3-BPG.
HbF HbA + 2.5 mM BPG 1.0 Given the oxygen dissociation curves, which of the HbA, HbF + following statements are correct? 2.5 mM BPG 0.8 HbA lysate O Purified HbA has a higher oxygen affinity than purified HbF. 0.6 2,3-BPG is an allosteric activator of HbA. The allosteric effects of 2,3-BPG are homotropic. 0.4 2,3-BPG only weakly interacts with HbF. HbF loads oxygen at lower pO, than does HbA in the 0.2 presence of 2,3-BPG.
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter18: Glycolysis
Section: Chapter Questions
Problem 17P
Related questions
Question
Given the oxygen dissociation curves, which of the following statements are correct?
Which residue in HbA β do you think contributes the most to the increased interaction between HbA aand 2,3‑BPG?
Transcribed Image Text:HbF
HbA + 2.5 mM BPG
1.0
Given the oxygen dissociation curves, which of the
HbA
НЬF +
following statements are correct?
2.5 mM BРG
0.8
`HbA lysate
O Purified HbA has a higher oxygen affinity than
purified HbF.
0.6
2,3-BPG is an allosteric activator of HbA.
The allosteric effects of 2,3-BPG are homotropic.
0.4
2,3-BPG only weakly interacts with HbF.
HbF loads oxygen at lower pO, than does HbA in the
0.2
presence of 2,3-BPG.
0 10
30
40
50
60
70
pO, (torr)
The researcher obtains the sequence used to generate the recombinant HbF and also sequences her sample of purified HbA.
Knowing that the HbA ß subunit C-terminus is important for the interaction between HbA and 2,3-BPG, the researcher
compares this portion of HbA with the C-terminal portion of the HbF y subunit.
Primary sequence data
НЬА В
136
GVANALAHKYH
146
HbF Y
136
AVASALSSRYH
146
The structure of 2,3-BPG is shown.
Which residue in HbA ß do you think contributes the
most to the increased interaction between HbA
aand 2,3-BPG?
2-
20
1/0,

Transcribed Image Text:Which residue in HbA ß do you think contributes the
most to the increased interaction between HbA
aand 2,3-BPG?
Lys-144
His-143
Asn-139
Ala-142
Gly-136
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning