Iculate the actual, physiological deltaG for the reaction Phosphocreatine + ADP > creatine + ATP at 35.61°C, as it occurs in the cylosol of 59 mM, creatine at 1.67 mM, ADP at 0.75 mM, and ATP at 2.49 mM. Give the answer in kJ/mol to one decimal place. The standard deltaG hydrolysis of phospho eurons, wiul p 72 Jllmel nnd tho ctandard deltaG for the nhosnhorylation of ADP with Pi is +35.05 kJ/mol. Give the answer in KJ/mol to one decimal place.
Iculate the actual, physiological deltaG for the reaction Phosphocreatine + ADP > creatine + ATP at 35.61°C, as it occurs in the cylosol of 59 mM, creatine at 1.67 mM, ADP at 0.75 mM, and ATP at 2.49 mM. Give the answer in kJ/mol to one decimal place. The standard deltaG hydrolysis of phospho eurons, wiul p 72 Jllmel nnd tho ctandard deltaG for the nhosnhorylation of ADP with Pi is +35.05 kJ/mol. Give the answer in KJ/mol to one decimal place.
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
any biochemistry expect. please help, it's my last attempt..
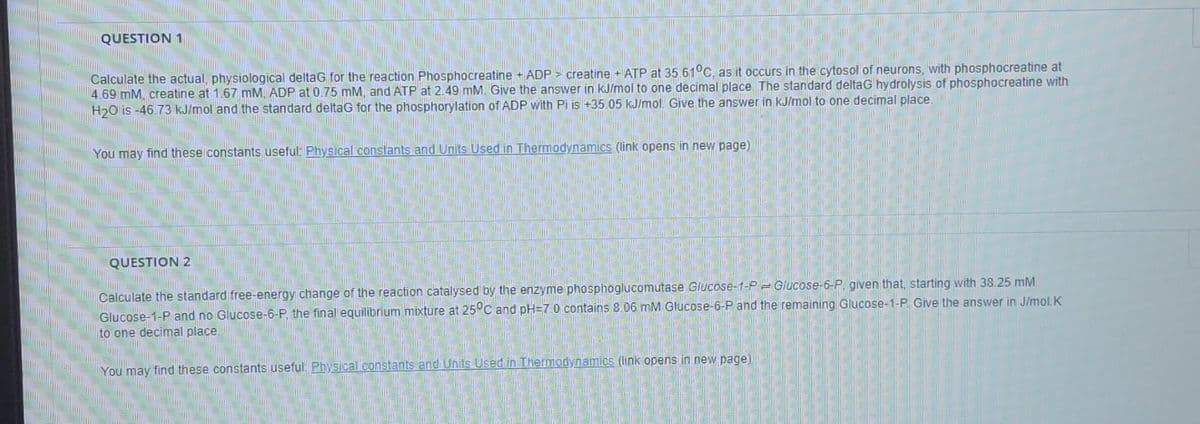
Transcribed Image Text:QUESTION 1
Calculate the actual, physiological deltaG for the reaction Phosphocreatine + ADP > creatine +ATP at 35.61°C, as it occurs in the cytosol of neurons, with phosphocreatine at
4.69 mM, creatine at 1.67 mM, ADP at 0.75 mM, and ATP at 2.49 mM. Give the answer in kJ/mol to one decimal place The standard deltaG hydrolysis of phosphocreatine with
H20 is -46.73 kJ/mol and the standard deltaG for the phosphorylation of ADP with Pi is +35.05 kJ/mol. Give the answer in kJ/mol to one decimal place.
You may find these constants useful: Physical constants and Units Used in Thermodynamics (link opens in new page)
QUESTION 2
Calculate the standard free-energy change of the reaction catalysed by the enzyme phosphoglucomutase Glucose-1-P Glucose-6-P, given that, starting with 38.25 mM
Glucose-1-P and no Glucose-6-P, the final equilibrium mixture at 25C and pH=7 0 contains 8.06 mM Glucose-6-P and the remaining Glucose-1-P. Give the answer in J/mol.K
to one decimal place.
You may find these constants useful: Physical constants and Units Used in Thermodynamics (link opens in new page)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON