Given that the reduction potential Eo'= -320, +10, +816, and +50mV for NAD+, fumarate, 02 and G3P DH bound FAD, respectively, calculate the free energy for a pair of electrons originating from the oxidation of glycerol-3-P to DHAP of, as it traverses the ETC. R = 8.315 x 10 J/mol K; Faraday constant, F= 96.48 KJ/V-mol, assume standard state T= 25°C and physiological conditions are T= 37°C. Report a whole number. Remember a Reduction potential is DEFINED as X(c) +e- --> X(red) Be sure you have defined your oxidation and reduction species properly.
Given that the reduction potential Eo'= -320, +10, +816, and +50mV for NAD+, fumarate, 02 and G3P DH bound FAD, respectively, calculate the free energy for a pair of electrons originating from the oxidation of glycerol-3-P to DHAP of, as it traverses the ETC. R = 8.315 x 10 J/mol K; Faraday constant, F= 96.48 KJ/V-mol, assume standard state T= 25°C and physiological conditions are T= 37°C. Report a whole number. Remember a Reduction potential is DEFINED as X(c) +e- --> X(red) Be sure you have defined your oxidation and reduction species properly.
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter20: Electron Transport And Oxidative Phosphorylation
Section: Chapter Questions
Problem 10P
Related questions
Question
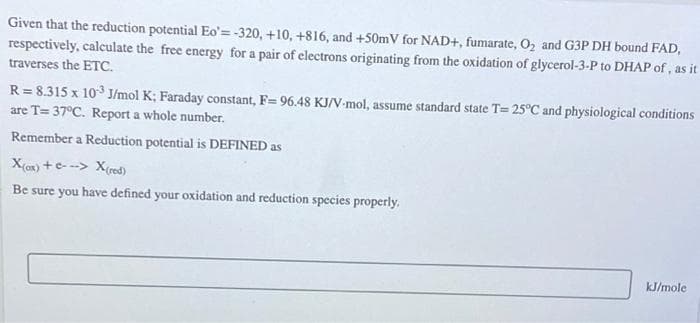
Transcribed Image Text:Given that the reduction potential Eo'=-320, +10, +816, and +50mV for NAD+, fumarate, 02 and G3P DH bound FAD,
respectively, calculate the free energy for a pair of electrons originating from the oxidation of glycerol-3-P to DHAP of, as it
traverses the ETC.
R = 8.315 x 10 J/mol K; Faraday constant, F= 96.48 KJ/V-mol, assume standard state T= 25°C and physiological conditions
are T= 37°C. Report a whole number.
Remember a Reduction potential is DEFINED as
X(ax) +e- --> X(red)
Be sure you have defined your oxidation and reduction species properly.
kJ/mole
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning