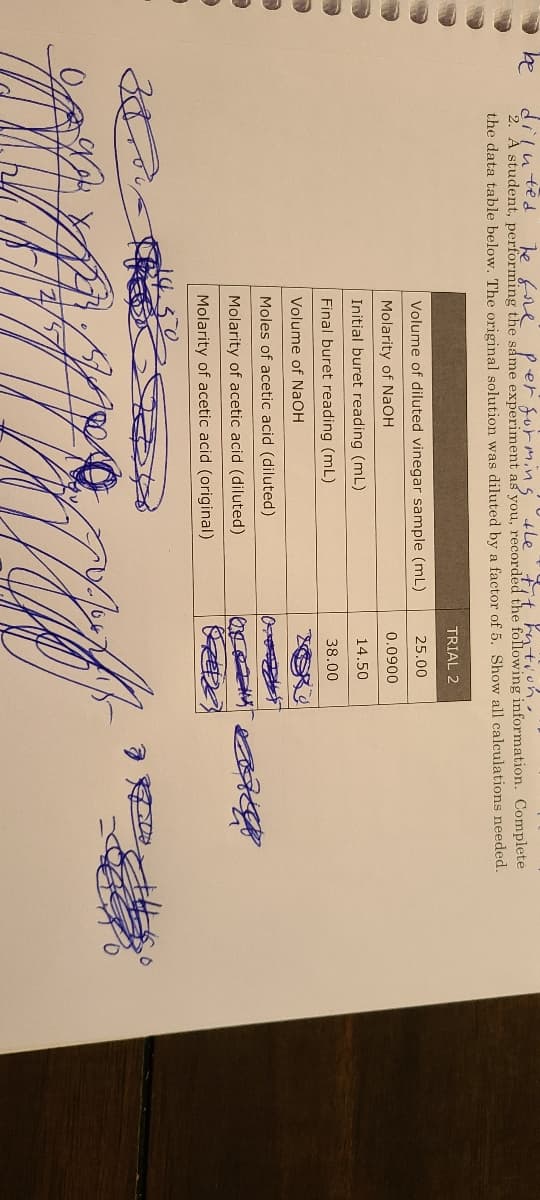
he fe 2. A student, performing the same experiment as you, recorded the following information. Complete the data table below. The original solution was diluted by a factor of 5. Show all calculations needed. TRIAL 2 Volume of diluted vinegar sample (mL) 25.00 Molarity of NaOH 0.0900 Initial buret reading (mL) 14.50 Final buret reading (mL) 38.00 Volume of NAOH Moles of acetic acid (diluted) Molarity of acetic acid (diluted) Molarity of acetic acid (original)
he fe 2. A student, performing the same experiment as you, recorded the following information. Complete the data table below. The original solution was diluted by a factor of 5. Show all calculations needed. TRIAL 2 Volume of diluted vinegar sample (mL) 25.00 Molarity of NaOH 0.0900 Initial buret reading (mL) 14.50 Final buret reading (mL) 38.00 Volume of NAOH Moles of acetic acid (diluted) Molarity of acetic acid (diluted) Molarity of acetic acid (original)
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.19QAP
Related questions
Question
Help I don't know how to do this
Transcribed Image Text:be
diluted
2. A student, performing the same experiment as you, recorded the following information. Complete
the data table below. The original solution was diluted by a factor of 5. Show all calculations needed.
Te fre P er surmins 4letit kntion.
TRIAL 2
Volume of diluted vinegar sample (mL)
25.00
Molarity of NAOH
0.0900
Initial buret reading (mL)
14.50
Final buret reading (mL)
38.00
Volume of NaOH
Moles of acetic acid (diluted)
Molarity of acetic acid (diluted)
Molarity of acetic acid (original)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

