What volume (mL) of 12 M HCl was required to react with the Mg? The data I have for you is this: Mg+2HCl-->MgCl2+H2 is the chemical equation. The mass of magnesium is 0.0273 grams and the moles is 0.001123 The moles of HCl that were consumed in the reaction was 0.002246 moles With this data, I need to know how we can get the volume (mL) of 12 M HCl was required to react with the Mg?
What volume (mL) of 12 M HCl was required to react with the Mg? The data I have for you is this: Mg+2HCl-->MgCl2+H2 is the chemical equation. The mass of magnesium is 0.0273 grams and the moles is 0.001123 The moles of HCl that were consumed in the reaction was 0.002246 moles With this data, I need to know how we can get the volume (mL) of 12 M HCl was required to react with the Mg?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 62QAP: Ten mL of concentrated H3PO4 (91.7% by mass, d=1.69g/mL) was accidentally poured into a beaker...
Related questions
Question
100%
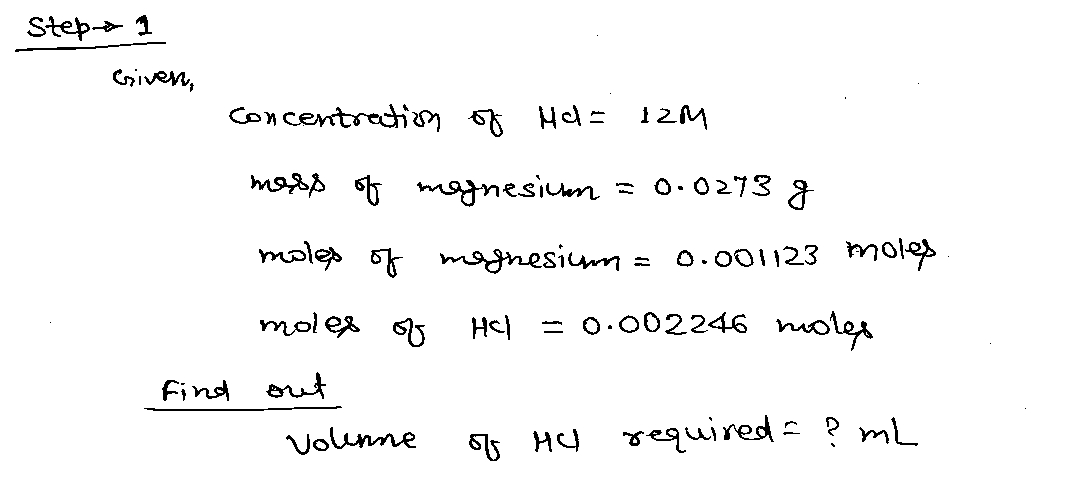
What volume (mL) of 12 M HCl was required to react with the Mg?
The data I have for you is this:
Mg+2HCl-->MgCl2+H2 is the chemical equation.
The mass of magnesium is 0.0273 grams and the moles is 0.001123
The moles of HCl that were consumed in the reaction was 0.002246 moles
With this data, I need to know how we can get the volume (mL) of 12 M HCl was required to react with the Mg?
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
