Assume that before separation, the pressure at the bottom of the milk bottle is pmix. How does the pressure at the bottom of the milk bottle after separation, psep, compare to Pmix ? For simplicity, you may assume that the weight and density of the cream is negligible compared to that of the milk. ► View Available Hint(s) O O Psep > Pmix Psep = Pmix Psep Pmix
Assume that before separation, the pressure at the bottom of the milk bottle is pmix. How does the pressure at the bottom of the milk bottle after separation, psep, compare to Pmix ? For simplicity, you may assume that the weight and density of the cream is negligible compared to that of the milk. ► View Available Hint(s) O O Psep > Pmix Psep = Pmix Psep Pmix
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 14E
Related questions
Question

Transcribed Image Text:Assume that before separation, the pressure at the bottom of the milk bottle is Pmix. How does the pressure at
the bottom of the milk bottle after separation, psep, compare to Pmix? For simplicity, you may assume that the
weight and density of the cream is negligible compared to that of the milk.
► View Available Hint(s)
Psep > Pmix
Psep = - Pmix
Psep Pmix
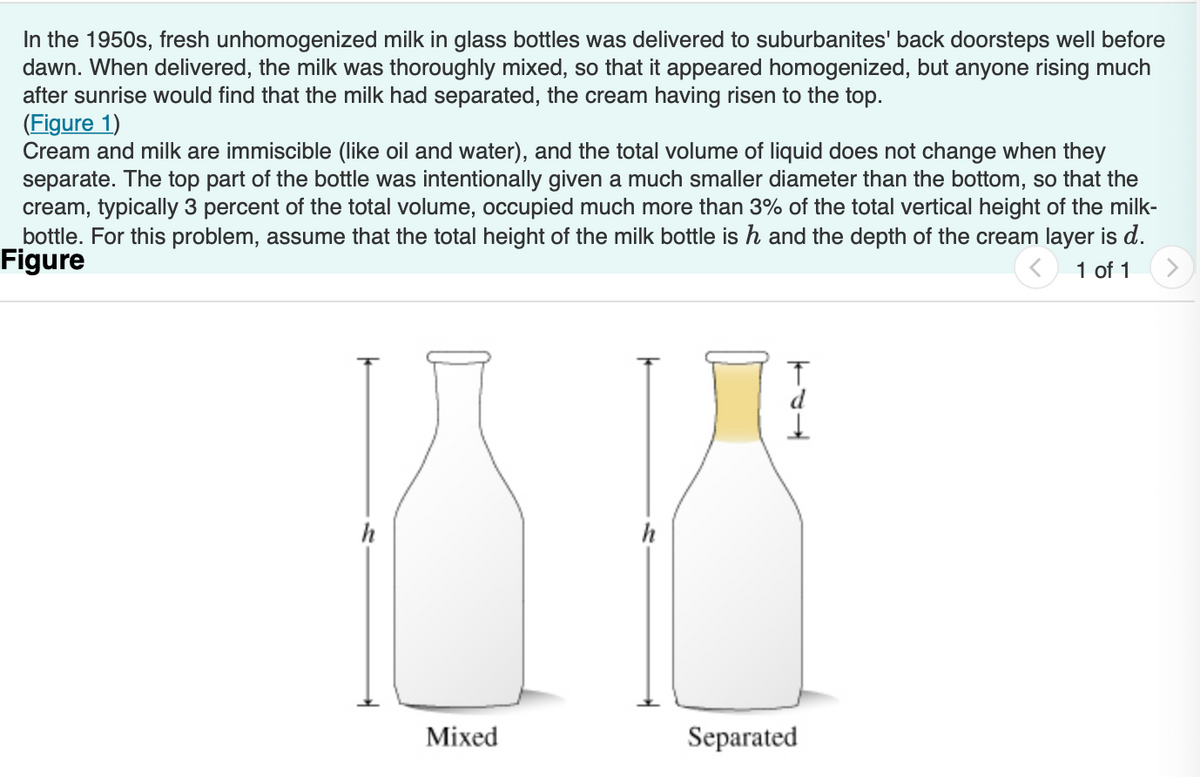
Transcribed Image Text:In the 1950s, fresh unhomogenized milk in glass bottles was delivered to suburbanites' back doorsteps well before
dawn. When delivered, the milk was thoroughly mixed, so that it appeared homogenized, but anyone rising much
after sunrise would find that the milk had separated, the cream having risen to the top.
(Figure 1)
Cream and milk are immiscible (like oil and water), and the total volume of liquid does not change when they
separate. The top part of the bottle was intentionally given a much smaller diameter than the bottom, so that the
cream, typically 3 percent of the total volume, occupied much more than 3% of the total vertical height of the milk-
bottle. For this problem, assume that the total height of the milk bottle is h and the depth of the cream layer is d.
Figure
1 of 1
h
Mixed
h
Separated
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning