You are asked to prepare a 1.000L solution of 45 M CHOs (glucose, molar mass 180 16 g/mol) in a lab by dissolving 811.0gof glucose in water Consider the following two scenarios in which you commit a user emor while preparing this solution Prepared in a volumetric flask Prepared in a beaker Assumed volume 1.000 L 1.000 L Volumetric Added water 2.0 em above the line, which comesponds to 63 ml. (D D063 La addonal soltion volume 8110 g takes up S26 ml of space rather than 500 ml of space error Preparation details You add the glucose to a volumetric flask and then add water unt dissolves. The water bote you are uning has You prepare the solution by adding gucese to a large beaker and appraximated the volume aworn tip, and you inadvertently add too much water such that the meniscus is above the ne The dameter of of the glucose to be 500 ml. Therefore, you add 500. ml of water to the 525 ml in the the neck of the volumetric fask is 229 cm beaker uning a graduated cylinder You decide to evaluate and compare the erors you made while preparing the solutions using the diferent methods Calculate the actval concentatons of the intended 45 M glucose solutions prepared by each methed based on their actual final volumes Express the concentrations in molarity to two decimal places. Make certain each field is complete before submitting your answer. View Available Hints) 811.0 g glucose enog gucose Concentration Concentration Pearson
You are asked to prepare a 1.000L solution of 45 M CHOs (glucose, molar mass 180 16 g/mol) in a lab by dissolving 811.0gof glucose in water Consider the following two scenarios in which you commit a user emor while preparing this solution Prepared in a volumetric flask Prepared in a beaker Assumed volume 1.000 L 1.000 L Volumetric Added water 2.0 em above the line, which comesponds to 63 ml. (D D063 La addonal soltion volume 8110 g takes up S26 ml of space rather than 500 ml of space error Preparation details You add the glucose to a volumetric flask and then add water unt dissolves. The water bote you are uning has You prepare the solution by adding gucese to a large beaker and appraximated the volume aworn tip, and you inadvertently add too much water such that the meniscus is above the ne The dameter of of the glucose to be 500 ml. Therefore, you add 500. ml of water to the 525 ml in the the neck of the volumetric fask is 229 cm beaker uning a graduated cylinder You decide to evaluate and compare the erors you made while preparing the solutions using the diferent methods Calculate the actval concentatons of the intended 45 M glucose solutions prepared by each methed based on their actual final volumes Express the concentrations in molarity to two decimal places. Make certain each field is complete before submitting your answer. View Available Hints) 811.0 g glucose enog gucose Concentration Concentration Pearson
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter11: Solutions And Colloids
Section: Chapter Questions
Problem 8E: Solutions of hydrogen in palladium may be formed by exposing Pd metal to H2 gas. The concentration...
Related questions
Question
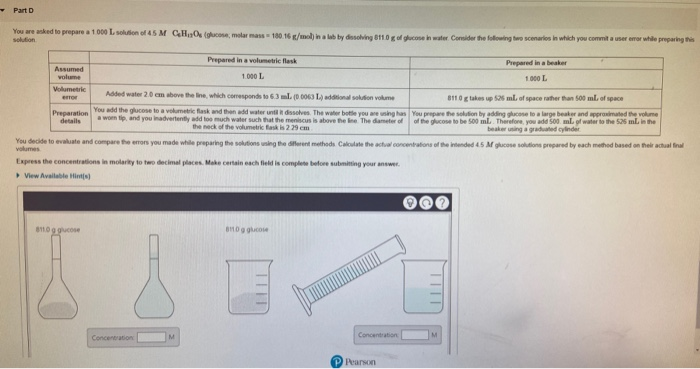
Transcribed Image Text:You are asked to prepare a 1.000L solution of 45 M CHOs (glucose, molar mass 180 16 g/mol) in a lab by dissolving 811.0gof glucose in water Consider the following two scenarios in which you commit a user emor while preparing this
solution
Prepared in a volumetric flask
Prepared in a beaker
Assumed
volume
1.000 L
1.000 L
Volumetric
Added water 2.0 em above the line, which comesponds to 63 ml. (D D063 La addonal soltion volume
8110 g takes up S26 ml of space rather than 500 ml of space
error
Preparation
details
You add the glucose to a volumetric flask and then add water unt dissolves. The water bote you are uning has You prepare the solution by adding gucese to a large beaker and appraximated the volume
aworn tip, and you inadvertently add too much water such that the meniscus is above the ne The dameter of of the glucose to be 500 ml. Therefore, you add 500. ml of water to the 525 ml in the
the neck of the volumetric fask is 229 cm
beaker uning a graduated cylinder
You decide to evaluate and compare the erors you made while preparing the solutions using the diferent methods Calculate the actval concentatons of the intended 45 M glucose solutions prepared by each methed based on their actual final
volumes
Express the concentrations in molarity to two decimal places. Make certain each field is complete before submitting your answer.
View Available Hints)
811.0 g glucose
enog gucose
Concentration
Concentration
Pearson
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning