How many milliliters of 0.592 M KOH are needed to react completely with 55.0 mL of 0.271 M FeCl2 solution to precipitate Fe(OH)2? The net ionic equation is: Fe2*(aq) + 20H (aq) → Fe(OH)2(s) VKOH = i ! ml
How many milliliters of 0.592 M KOH are needed to react completely with 55.0 mL of 0.271 M FeCl2 solution to precipitate Fe(OH)2? The net ionic equation is: Fe2*(aq) + 20H (aq) → Fe(OH)2(s) VKOH = i ! ml
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section3.11: Stoichiometry In Aqueous Solutions
Problem 3.24PSP
Related questions
Question
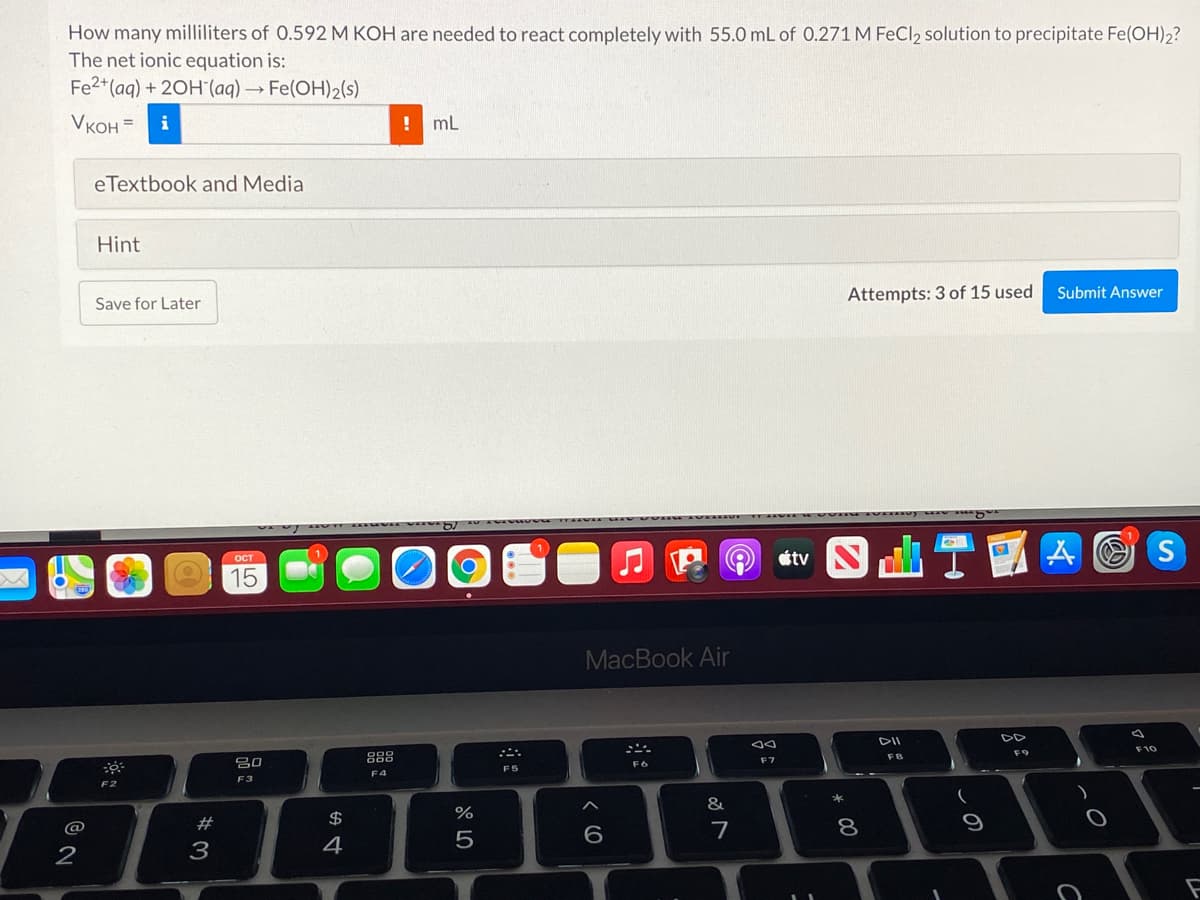
Transcribed Image Text:How many milliliters of 0.592 M KOH are needed to react completely with 55.0 mL of 0.271 M FeCl2 solution to precipitate Fe(OH)2?
The net ionic equation is:
Fe2*(aq) + 20H (aq) → Fe(OH)2(s)
VKOH =
i
! mL
eTextbook and Media
Hint
Attempts: 3 of 15 used
Submit Answer
Save for Later
ост
tv
15
MacBook Air
DII
DD
F9
888
80
F5
F4
F3
F2
&
@
#
$
7
8
6.
2
3
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning