How many milliliters of 0.592 M KOH are needed to react completely with 55.0 mL of 0.271 M FeCl2 solution to precipitate Fe(OH)2? The net ionic equation is: Fe2*(aq) + 20H'(aq) → Fe(OH)2(s) VKOH = i mL
How many milliliters of 0.592 M KOH are needed to react completely with 55.0 mL of 0.271 M FeCl2 solution to precipitate Fe(OH)2? The net ionic equation is: Fe2*(aq) + 20H'(aq) → Fe(OH)2(s) VKOH = i mL
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 95AP: 95. Many metal ions form insoluble sulfide compounds when a solution of the metal ion is treated...
Related questions
Question
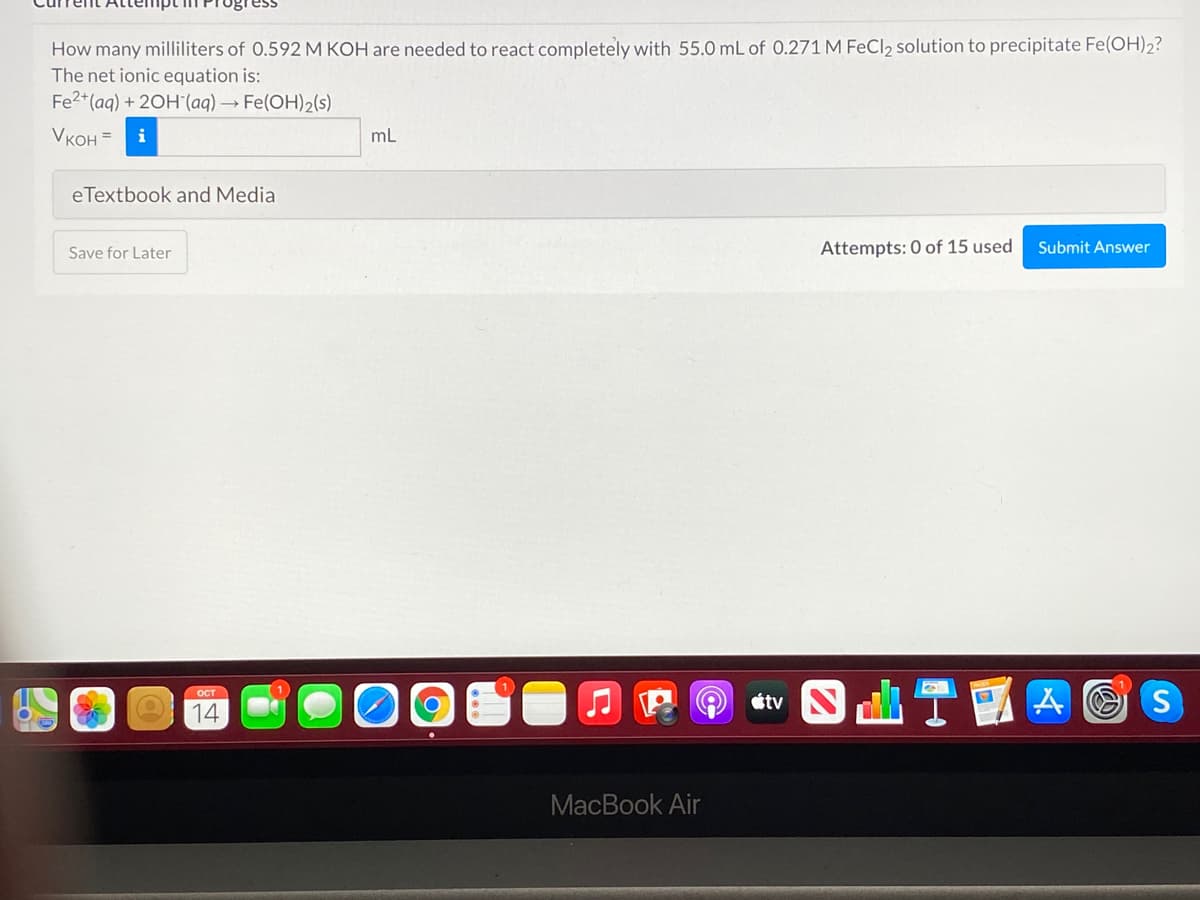
Transcribed Image Text:How many milliliters of 0.592 M KOH are needed to react completely with 55.0 mL of 0.271 M FeCl2 solution to precipitate Fe(OH)2?
The net ionic equation is:
Fe2+(aq) + 20H(aq) → Fe(OH)2(s)
VKOH =
i
mL
eTextbook and Media
Save for Later
Attempts: 0 of 15 used
Submit Answer
A O S
OCT
étv
14
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning