凸https://ng.cengage.com/static/nb/ui/evo/index.html? ☆ ☆ DTAP Q Search this course ons Use the References to access important values if needed for this question. The activation energy of the gas-phase reaction 2NOCIg)2NO(g) +Ch(g) is 97.9 kJ mol-1, and the change in the internal energy in the reaction is ΔΕ kJ mol. Calculate the activation energy of the reaction 69.0 2N0(g) +Cl2(g) 2NOCİ(g) kJ mol1 Submit Answer 3 question attempts remaining Back Next O Type here to search ove
凸https://ng.cengage.com/static/nb/ui/evo/index.html? ☆ ☆ DTAP Q Search this course ons Use the References to access important values if needed for this question. The activation energy of the gas-phase reaction 2NOCIg)2NO(g) +Ch(g) is 97.9 kJ mol-1, and the change in the internal energy in the reaction is ΔΕ kJ mol. Calculate the activation energy of the reaction 69.0 2N0(g) +Cl2(g) 2NOCİ(g) kJ mol1 Submit Answer 3 question attempts remaining Back Next O Type here to search ove
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter18: Chemical Kinetics
Section: Chapter Questions
Problem 36P
Related questions
Question
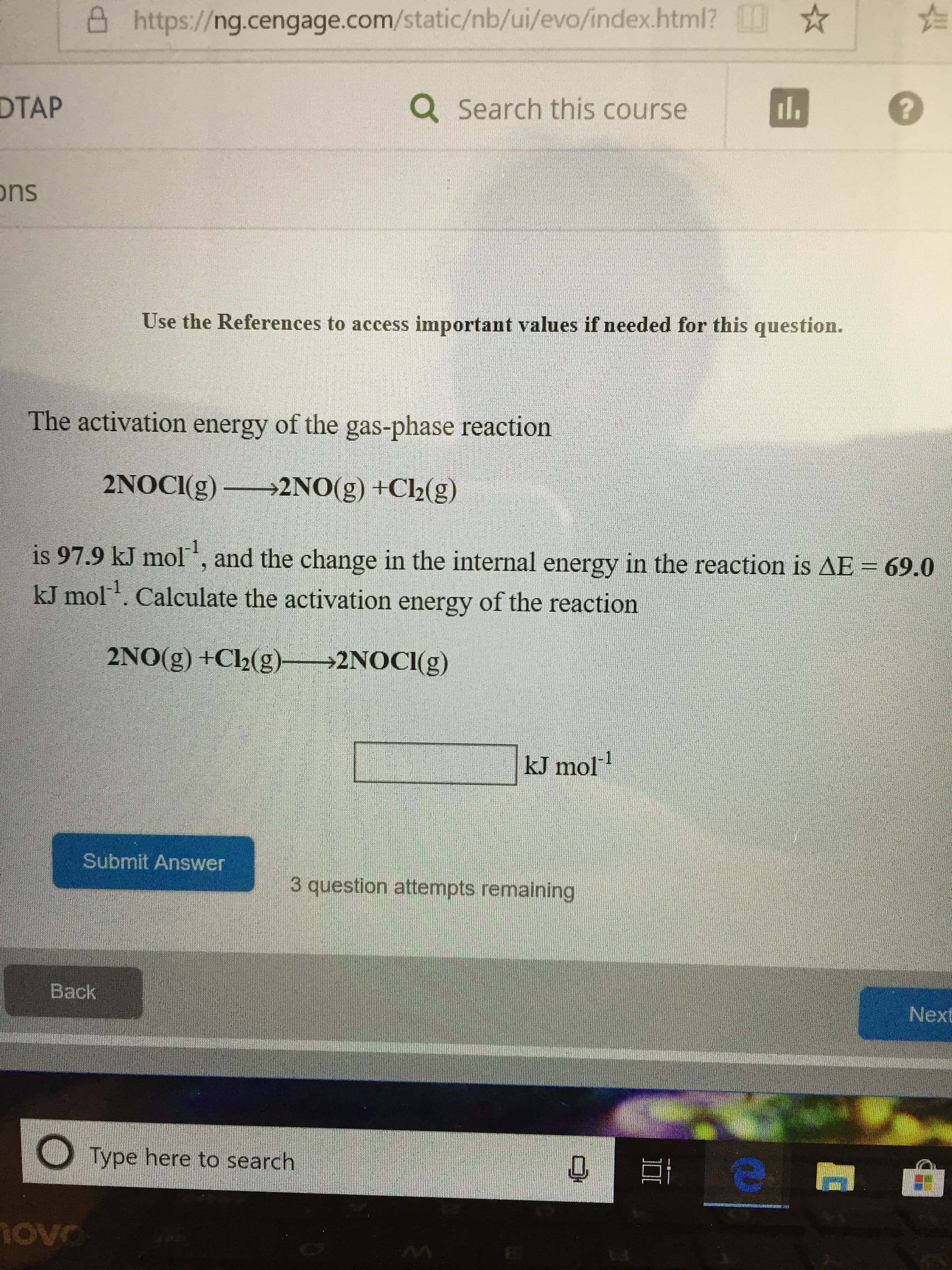
Transcribed Image Text:凸https://ng.cengage.com/static/nb/ui/evo/index.html? ☆ ☆
DTAP
Q Search this course
ons
Use the References to access important values if needed for this question.
The activation energy of the gas-phase reaction
2NOCIg)2NO(g) +Ch(g)
is 97.9 kJ mol-1, and the change in the internal energy in the reaction is ΔΕ
kJ mol. Calculate the activation energy of the reaction
69.0
2N0(g) +Cl2(g)
2NOCİ(g)
kJ mol1
Submit Answer
3 question attempts remaining
Back
Next
O Type here to search
ove
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning