For the gas phase decomposition of t-butyl acetate, CH3COOC(CH3)3 →(CH3)½C=CH2 + CH3COOH the rate constant has been determined at several temperatures. When In k in s*' is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -2.04×10* K and a y-intercept of 30.7. The activation energy for the gas phase decomposition of t-butyl acetate is kJ/mol.
For the gas phase decomposition of t-butyl acetate, CH3COOC(CH3)3 →(CH3)½C=CH2 + CH3COOH the rate constant has been determined at several temperatures. When In k in s*' is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -2.04×10* K and a y-intercept of 30.7. The activation energy for the gas phase decomposition of t-butyl acetate is kJ/mol.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter13: Rates Of Reaction
Section: Chapter Questions
Problem 13.81QP: The following values of the rate constant were obtained for the decomposition of nitrogen dioxide at...
Related questions
Question
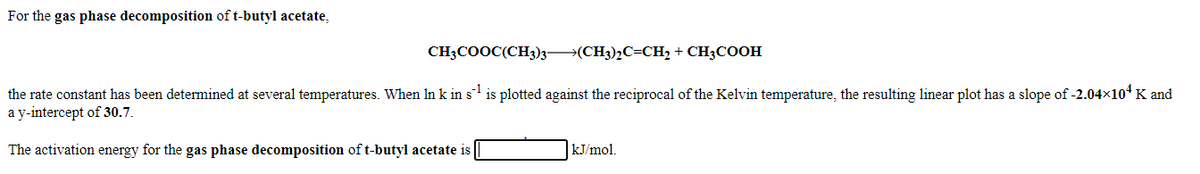
Transcribed Image Text:For the gas phase decomposition of t-butyl acetate,
CH3COOC(CH3)3→(CH3)2C=CH2 + CH3COOH
the rate constant has been determined at several temperatures. When In k in s is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -2.04x10* K and
a y-intercept of 30.7.
The activation energy for the gas phase decomposition of t-butyl acetate is
kJ/mol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning



General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning



Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning