Hydrogen peroxide (H;O2) decamposes into water and owygen. H,O;(ag) + H0(1) + 1/2 0,(a) Ordinarily, this reaction proceeds rather slowty. hut in the presence of some iodide ions (I , the decompositicn is mach faster. The decomposition in the peesence of indide was studied at 20 C, and the data were plotted in various ways. Lise the graphs helow to answer the questions that follow. M (mol/L) 1/M (L/mol) 0. -0.5 014 1. 0.6 0.5 5- 04 -1.5- 03 3- 024 2- Tlme (s) Tlme (s) Tlme (s) 100 300 600 100 290 500 000 600 a What is the order of reaction for the decomposition of hydrogen peroxide? O zero-onder O first-order O second-ceder b. Find the numerical value of the rate constant at 20C, including the correct units. (Enter an uneounded value.) k= c. Ckeain an estimate of the initial rate of reaction in the experiment that penduced the graphs (i.e, the rate at e= 0 in the graphs) (Enter an unrounded value.) rate = Jmol · L
Hydrogen peroxide (H;O2) decamposes into water and owygen. H,O;(ag) + H0(1) + 1/2 0,(a) Ordinarily, this reaction proceeds rather slowty. hut in the presence of some iodide ions (I , the decompositicn is mach faster. The decomposition in the peesence of indide was studied at 20 C, and the data were plotted in various ways. Lise the graphs helow to answer the questions that follow. M (mol/L) 1/M (L/mol) 0. -0.5 014 1. 0.6 0.5 5- 04 -1.5- 03 3- 024 2- Tlme (s) Tlme (s) Tlme (s) 100 300 600 100 290 500 000 600 a What is the order of reaction for the decomposition of hydrogen peroxide? O zero-onder O first-order O second-ceder b. Find the numerical value of the rate constant at 20C, including the correct units. (Enter an uneounded value.) k= c. Ckeain an estimate of the initial rate of reaction in the experiment that penduced the graphs (i.e, the rate at e= 0 in the graphs) (Enter an unrounded value.) rate = Jmol · L
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.52PAE: Hydrogen peroxide (H20i) decomposes into water and oxygen: H,O2(aq) — H,O(f) + ^O2(g) Ordinarily...
Related questions
Question
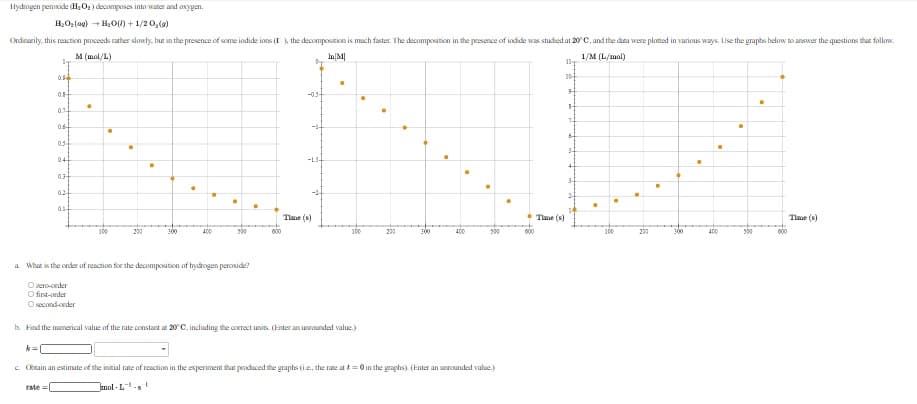
Transcribed Image Text:Hydrogen peroxide (H;O;) decomposes into water and awygen.
H,O;(a9) + H0(1) + 1/2 0,(a)
Ordinarily, this reaction proceeds rather slowty, but in the presence of some iodide ions (I ), the decomposition is much faster. The decomposition in the peesence of indide was studied at 20 C, and the data were plotted in various ways. Lise the graphs helow to answer the questions that follow.
M (mol/L)
1/M (L/mal)
0.
-0.5
0.14
06
--
0.5
5-
044
-1.5-
0.3
3-
024
2-
Tlme (s)
Tlme (s)
Tlme (s)
100
300
000
100
000
100
30
00
a What is the order of reaction for the decomposition of hydrogen peroxide?
O zero-oder
O first-onder
Osecond-ceder
b. Find the numerical value of the rate constant at 20'C, including the correct units. (Enter an uneounded value.)
k=
C. Cbeain an estimate of the initial rate of reaction in the experiment that penduced the graphs (i.e, the rate at = 0 in the graphs) (Enter an unrounded value.)
rate =
Jmol - L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning