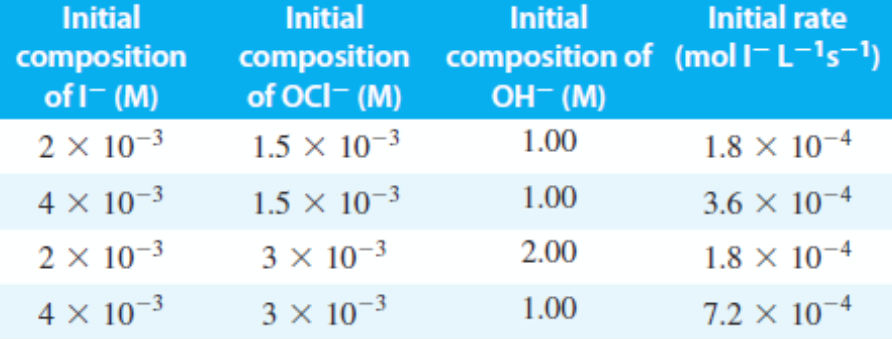
The kinetics of the reaction: I⁻ + OCl⁻ ⇄ OI⁻ + Cl⁻ was studied in basic aqueous media by Chia and Connick . The initial rate of I⁻ disappearance is given below for mixtures of various initial compositions, at 25°C (None of the solutions initially contained OI⁻ or Cl⁻ ). If the mechanism of the reaction is given below, which constants below play role in the reaction rate law: OCl⁻ + H₂O ⇄ HOCl + OH⁻ (rate constant=k₁, equil.const=K₁)---fast, equilibrium I⁻ + HOCl → HOI + Cl⁻ (rateconstant=k₂)---slow HOI + OH⁻ ⇄ H₂O + OI⁻ (rate constant=k₃, equil.const=K₂)---fast, equilibrium
The kinetics of the reaction: I⁻ + OCl⁻ ⇄ OI⁻ + Cl⁻ was studied in basic aqueous media by Chia and Connick . The initial rate of I⁻ disappearance is given below for mixtures of various initial compositions, at 25°C (None of the solutions initially contained OI⁻ or Cl⁻ ). If the mechanism of the reaction is given below, which constants below play role in the reaction rate law: OCl⁻ + H₂O ⇄ HOCl + OH⁻ (rate constant=k₁, equil.const=K₁)---fast, equilibrium I⁻ + HOCl → HOI + Cl⁻ (rateconstant=k₂)---slow HOI + OH⁻ ⇄ H₂O + OI⁻ (rate constant=k₃, equil.const=K₂)---fast, equilibrium
Chapter33: High-performance Liquid Chromatography
Section: Chapter Questions
Problem 33.14QAP
Related questions
Question
The kinetics of the reaction: I⁻ + OCl⁻ ⇄ OI⁻ + Cl⁻ was studied in basic aqueous media by Chia and Connick . The initial rate of I⁻ disappearance is given below for mixtures of various initial compositions, at 25°C (None of the solutions initially contained OI⁻ or Cl⁻ ).
If the mechanism of the reaction is given below, which constants below play role in the reaction rate law: OCl⁻ + H₂O ⇄ HOCl + OH⁻ (rate constant=k₁, equil.const=K₁)---fast, equilibrium I⁻ + HOCl → HOI + Cl⁻ (rateconstant=k₂)---slow HOI + OH⁻ ⇄ H₂O + OI⁻ (rate constant=k₃, equil.const=K₂)---fast, equilibrium
Transcribed Image Text:Initial
Initial
Initial
Initial rate
composition
of I- (M)
2 × 10–3
composition composition of (moll-L-1s-1)
of OCI- (M)
OH- (M)
1.5 x 10-3
1.00
1.8 × 10-4
4 x 10-3
1.5 × 10-3
1.00
3.6 × 10-4
2 × 10-3
3 × 10-3
2.00
1.8 X 10-4
4 x 10-3
3 x 10-3
1.00
7.2 × 10¬4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning