(i) Deduce the energy difference betweetn the conformers of compound X by referring to the table below. H,C- Compound X Support your answer hy drawing the conformer(s) involved, and show the calculations to obtain the energy difference. Energy (kJ mol"') 6.0 Energy cost by: He»CH3 eclipsed 1,3-diaxial (He>CH,) strain 1,3-diaxial (CH;»CH;) strain 3.8 15.4 (ii) Identify which of these conformers give the most and the least stable conformations.
(i) Deduce the energy difference betweetn the conformers of compound X by referring to the table below. H,C- Compound X Support your answer hy drawing the conformer(s) involved, and show the calculations to obtain the energy difference. Energy (kJ mol"') 6.0 Energy cost by: He»CH3 eclipsed 1,3-diaxial (He>CH,) strain 1,3-diaxial (CH;»CH;) strain 3.8 15.4 (ii) Identify which of these conformers give the most and the least stable conformations.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter6: Alkanes & Alkenes
Section: Chapter Questions
Problem 37CTQ: Indicate the relationship between each pair. Choose from: configurational stereoisomers,conformers,...
Related questions
Question
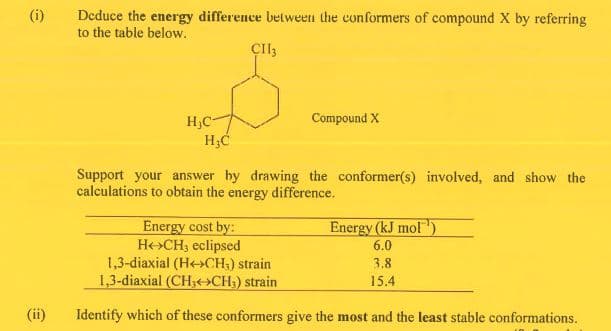
Transcribed Image Text:Deduce the energy difference betweetn the conformers of compound X by referring
to the table below.
(i)
ÇII3
Compound X
H,C
H;C
Support your answer hy drawing the conformer(s) involved, and show the
calculations to obtain the energy difference.
Energy cost by:
He>CH3 eclipsed
1,3-diaxial (He>CH,) strain
1,3-diaxial (CH3>CH;) strain
Energy (kJ mol)
6.0
3.8
15.4
(ii)
Identify which of these conformers give the most and the least stable conformations.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
