Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter16: Spontaneity Of Reaction
Section: Chapter Questions
Problem 32QAP
Related questions
Question
I need help with question 80?
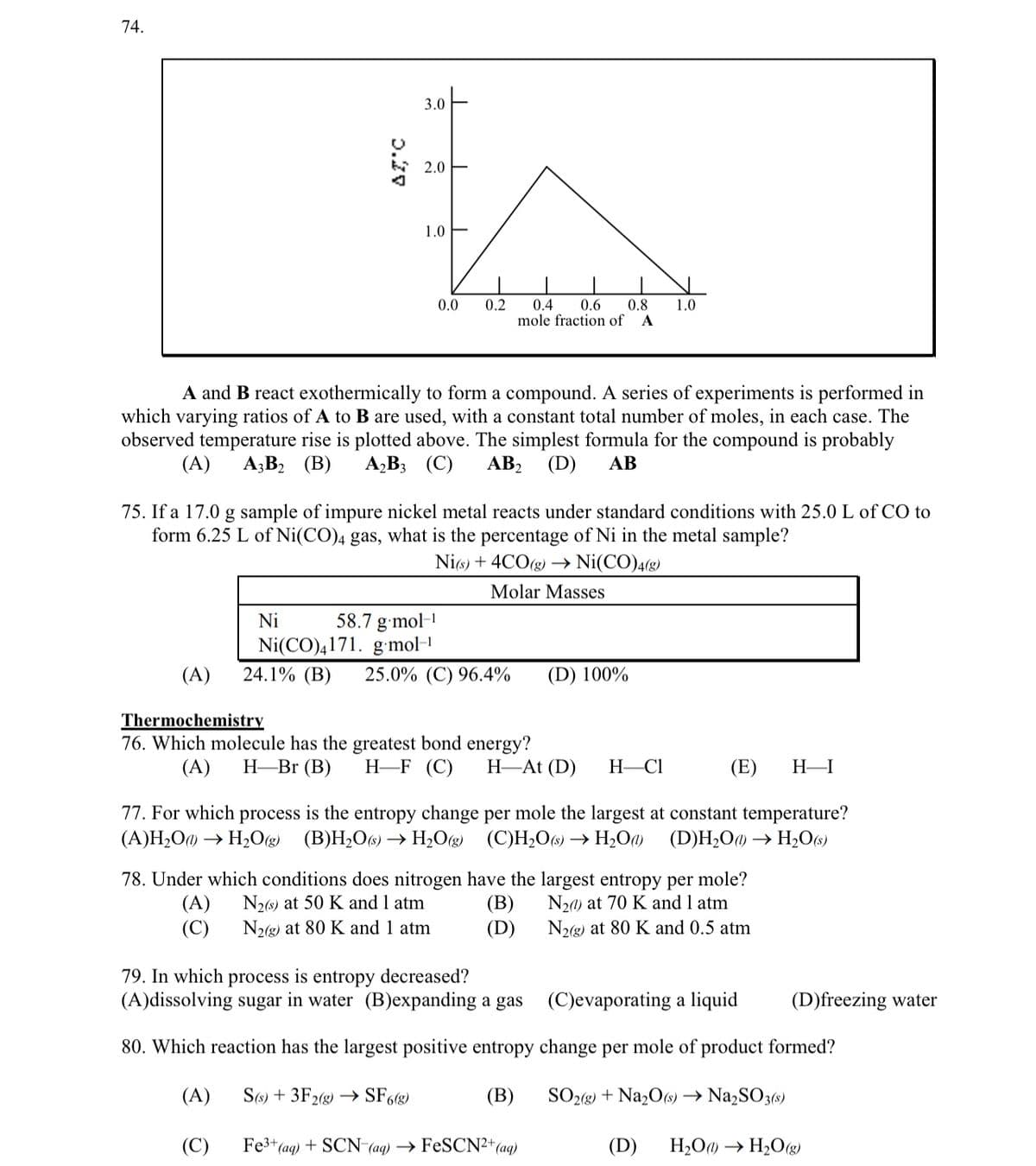
Transcribed Image Text:74.
(A)
AT, C
3.0
Ni
2.0
1.0
T
0.0
A and B react exothermically to form a compound. A series of experiments is performed in
which varying ratios of A to B are used, with a constant total number of moles, in each case. The
observed temperature rise is plotted above. The simplest formula for the compound is probably
(A) A3B₂ (B) A₂B3 (C) AB₂ (D) AB
0.2
75. If a 17.0 g sample of impure nickel metal reacts under standard conditions with 25.0 L of CO to
form 6.25 L of Ni(CO)4 gas, what is the percentage of Ni in the metal sample?
Ni(s) + 4CO(g) →→ Ni(CO)4(g)
Molar Masses
58.7 g.mol-¹
Ni(CO)4171. g.mol-1
24.1% (B) 25.0% (C) 96.4%
Fe3+(
0.4 0.6 0.8 1.0
mole fraction of A
Thermochemistry
76. Which molecule has the greatest bond energy?
(A) H-Br (B) H-F (C) H-At (D) H-Cl
(E) H-I
77. For which process is the entropy change per mole the largest at constant temperature?
(A)H₂O) → H₂O(g) (B)H₂O(s)→ H₂O(g) (C)H₂O(s) → H₂O) (D)H₂O) → H₂O(s)
78. Under which conditions does nitrogen have the largest entropy per mole?
(A) N₂(s) at 50 K and 1 atm
N₂) at 70 K and 1 atm
(C)
N2(g) at 80 K and 1 atm
N2(g) at 80 K and 0.5 atm
(B)
(D)
(D) 100%
79. In which process is entropy decreased?
(A)dissolving sugar in water (B)expanding a gas
80. Which reaction has the largest positive entropy
S(s) + 3F2(g) → SF6(g)
(A)
(B)
(C)
(aq) + SCN (aq) → FeSCN²+ (aq)
(C)evaporating a liquid
change per mole of product formed?
SO2(g) + Na₂O(s) → Na₂SO3(s)
(D) H₂O) → H₂O(g)
(D)freezing water
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning