I Review I Constants I Periodic Table Instant cold packs, often used to ice athletic injuries on the field, contain ammonium nitrate and water separated by a thin plastic divider. When the divider is broken, the ammonium nitrate dissolves according to the following endothermic reaction: NHẠNO3 (s) → NH;(aq) + NO3 (ag) Part A Calculate the change in enthalpy for the reaction. (Use 1.0 g/mL as the density of the solution and 4.18 J/(g.°C) as the specific heat capacity.) Express the enthalpy change in kilojoules per mole to two significant figures. In order to measure the enthalpy change for this reaction, 1.25 g of NH4NO3 is dissolved in enough water to make 25.0 mL of solution. The initial temperature is 25.8 ° C and the final temperature (after the solid dissolves) is 21.9 ° C. Bνα ΑΣΦ You may want to reference (Pages 385 - 387) Section 9.7 while completing this problem. ΔΗΚn kJ/mol Request Answer Submit
I Review I Constants I Periodic Table Instant cold packs, often used to ice athletic injuries on the field, contain ammonium nitrate and water separated by a thin plastic divider. When the divider is broken, the ammonium nitrate dissolves according to the following endothermic reaction: NHẠNO3 (s) → NH;(aq) + NO3 (ag) Part A Calculate the change in enthalpy for the reaction. (Use 1.0 g/mL as the density of the solution and 4.18 J/(g.°C) as the specific heat capacity.) Express the enthalpy change in kilojoules per mole to two significant figures. In order to measure the enthalpy change for this reaction, 1.25 g of NH4NO3 is dissolved in enough water to make 25.0 mL of solution. The initial temperature is 25.8 ° C and the final temperature (after the solid dissolves) is 21.9 ° C. Bνα ΑΣΦ You may want to reference (Pages 385 - 387) Section 9.7 while completing this problem. ΔΗΚn kJ/mol Request Answer Submit
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.102PAE: 9.102 A runner generates 418 kJ of energy per kilometer from the cellular oxidation of food. The...
Related questions
Question
100%
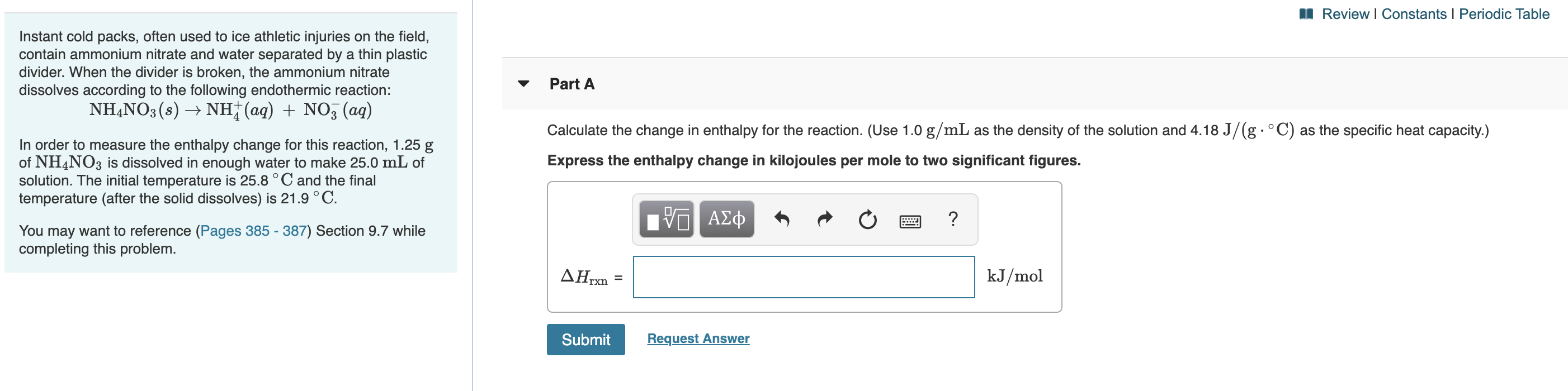
Transcribed Image Text:I Review I Constants I Periodic Table
Instant cold packs, often used to ice athletic injuries on the field,
contain ammonium nitrate and water separated by a thin plastic
divider. When the divider is broken, the ammonium nitrate
dissolves according to the following endothermic reaction:
NHẠNO3 (s) → NH;(aq) + NO3 (ag)
Part A
Calculate the change in enthalpy for the reaction. (Use 1.0 g/mL as the density of the solution and 4.18 J/(g.°C) as the specific heat capacity.)
Express the enthalpy change in kilojoules per mole to two significant figures.
In order to measure the enthalpy change for this reaction, 1.25 g
of NH4NO3 is dissolved in enough water to make 25.0 mL of
solution. The initial temperature is 25.8 ° C and the final
temperature (after the solid dissolves) is 21.9 ° C.
Bνα ΑΣΦ
You may want to reference (Pages 385 - 387) Section 9.7 while
completing this problem.
ΔΗΚn
kJ/mol
Request Answer
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,