I Review | Constants | Periodic Table lonone is a compound that gives violets their aroma. The small, edible, purple flowers of violets are used in salads and to make teas. Liquid ionone has a density of 0.935 g/mL. Submit Request Answer Part C If the molecular formula of ionone is C13H200, what is the molar mass of ionone? Express your answer to two decimal places and include the appropriate units. lonone HA 1] ? Molar mass = Value Units Submit Request Answer Part D How many moles are in 3.91 mL of ionone? Express your answer to three significant figures and include the appropriate units. HA Value Units Submit Request Answer Provide Feedback Next >
I Review | Constants | Periodic Table lonone is a compound that gives violets their aroma. The small, edible, purple flowers of violets are used in salads and to make teas. Liquid ionone has a density of 0.935 g/mL. Submit Request Answer Part C If the molecular formula of ionone is C13H200, what is the molar mass of ionone? Express your answer to two decimal places and include the appropriate units. lonone HA 1] ? Molar mass = Value Units Submit Request Answer Part D How many moles are in 3.91 mL of ionone? Express your answer to three significant figures and include the appropriate units. HA Value Units Submit Request Answer Provide Feedback Next >
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter5: Resonance
Section: Chapter Questions
Problem 18E
Related questions
Question
Transcribed Image Text:O Course Home
b Chemistry Question | bartleby
G indivuials with austium good to
O X
->
A openvellum.ecollege.com/course.html?courseld=16561285&OpenVellumHMAC=446c877376a9bc7ac878d8ad119f4717#10001
E Apps
G Gmail
YouTube
O Maps
E Connect - To Do As..
O OCCC Moodle
P chem work
b help
Balance Chemical E.
Course Home
Problem Cl.26 - Copy
>
12 of 13
Syllabus
I Review | Constants | Periodic Table
Scores
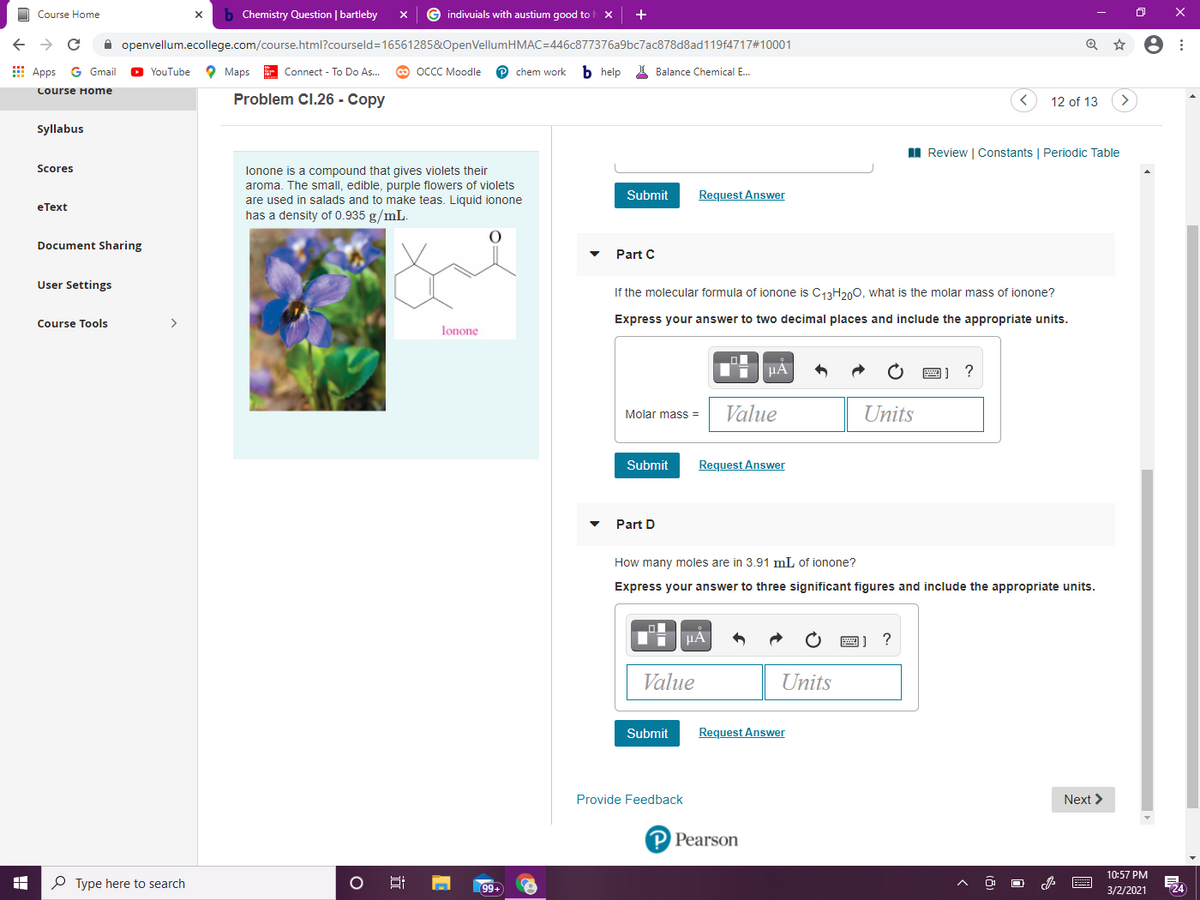
lonone is a compound that gives violets their
aroma. The small, edible, purple flowers of violets
are used in salads and to make teas. Liquid ionone
has a density of 0.935 g/mL.
Submit
Request Answer
eТext
Document Sharing
Part C
User Settings
If the molecular formula of ionone is C13H200, what is the molar mass of ionone?
Course Tools
>
Express your answer to two decimal places and include the appropriate units.
Ionone
Value
Units
Molar mass =
Submit
Request Answer
Part D
How many moles are in 3.91 mL of ionone?
Express your answer to three significant figures and include the appropriate units.
HA
Value
Units
Submit
Request Answer
Provide Feedback
Next >
P Pearson
10:57 PM
P Type here to search
99+
24
3/2/2021
近
Transcribed Image Text:O Course Home
b Chemistry Question | bartleby
G indivuials with austium good to
->
A openvellum.ecollege.com/course.html?courseld=16561285&OpenVellumHMAC=446c877376a9bc7ac878d8ad119f4717#10001
Q *
E Apps G Gmail
YouTube
о Марs
E Connect - To Do As...
O OCCC Moodle
P chem work
b help
Balance Chemical E.
Syllabus
II Review | Constants | Periodic Table
Scores
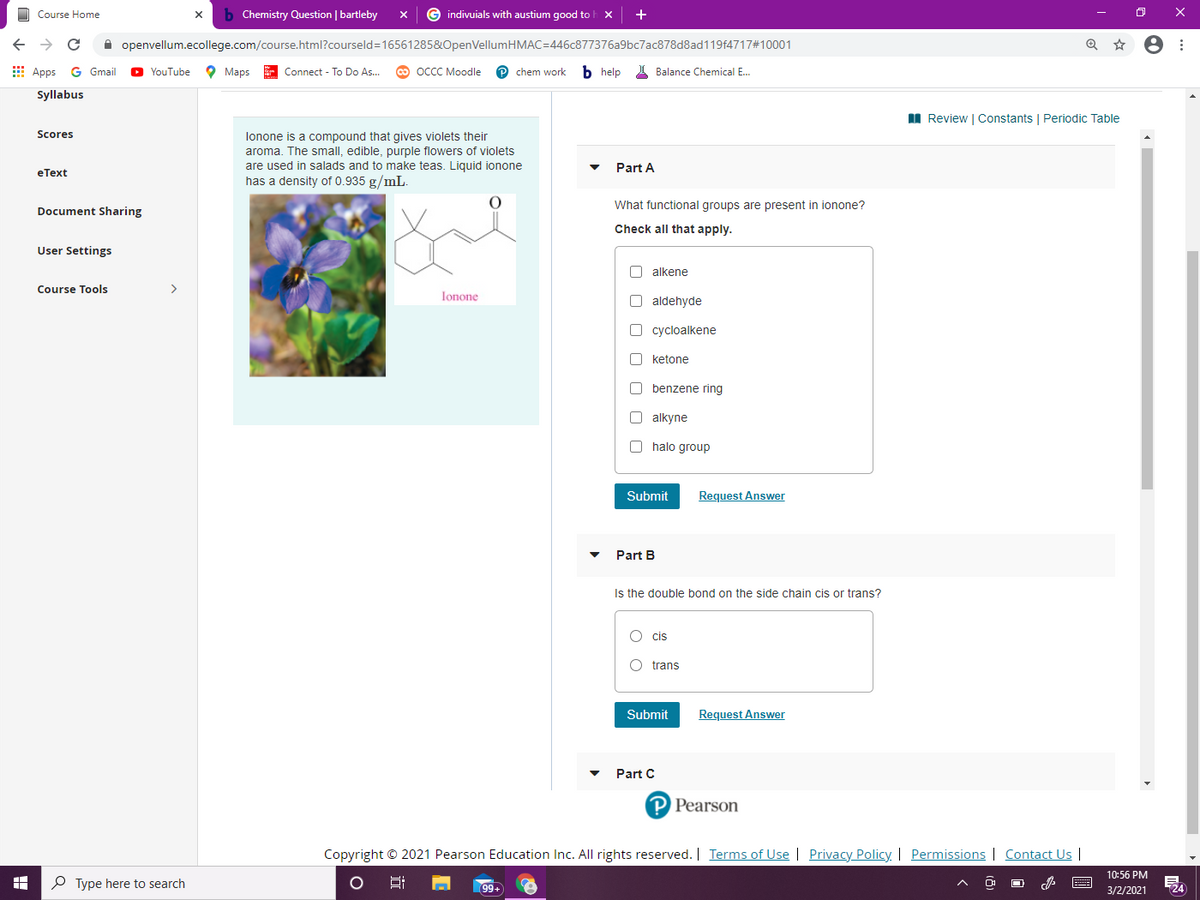
lonone is a compound that gives violets their
aroma. The small, edible, purple flowers of violets
are used in salads and to make teas. Liquid ionone
has a density of 0.935 g/mL.
Part A
еТext
What functional groups are present in ionone?
Document Sharing
Check all that apply.
User Settings
O alkene
Course Tools
Ionone
O aldehyde
cycloalkene
O ketone
O benzene ring
O alkyne
O halo group
Submit
Request Answer
Part B
Is the double bond on the side chain cis or trans?
O cis
O trans
Submit
Request Answer
Part C
P Pearson
Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy| Permissions | Contact Us |
10:56 PM
P Type here to search
99+
24
3/2/2021
O O O O O O O
近
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
