Liquid hexane (CH3(CH2) CH3will react with gaseous oxygen (02) to produce gaseous carbon dioxide (CO2) and gaseous water (H2O). Suppose 7.76 g 4 of hexane is mixed with 19. g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. x10 ? Check Explanation Privacy Terms of Use 2019 McGraw Hill Education All Rights Reserved. Sta8228.txt Sta8230.txt Sta5429.txt Gases lab repo....pdf Alyssa Flores R....pdf MacBook Pro Dil F 10 F9 F8 E7 F6 F5 F4 F3 F2 X రు
Liquid hexane (CH3(CH2) CH3will react with gaseous oxygen (02) to produce gaseous carbon dioxide (CO2) and gaseous water (H2O). Suppose 7.76 g 4 of hexane is mixed with 19. g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. x10 ? Check Explanation Privacy Terms of Use 2019 McGraw Hill Education All Rights Reserved. Sta8228.txt Sta8230.txt Sta5429.txt Gases lab repo....pdf Alyssa Flores R....pdf MacBook Pro Dil F 10 F9 F8 E7 F6 F5 F4 F3 F2 X రు
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter1: Chemistry And Measurement
Section: Chapter Questions
Problem 1.153QP
Related questions
Question
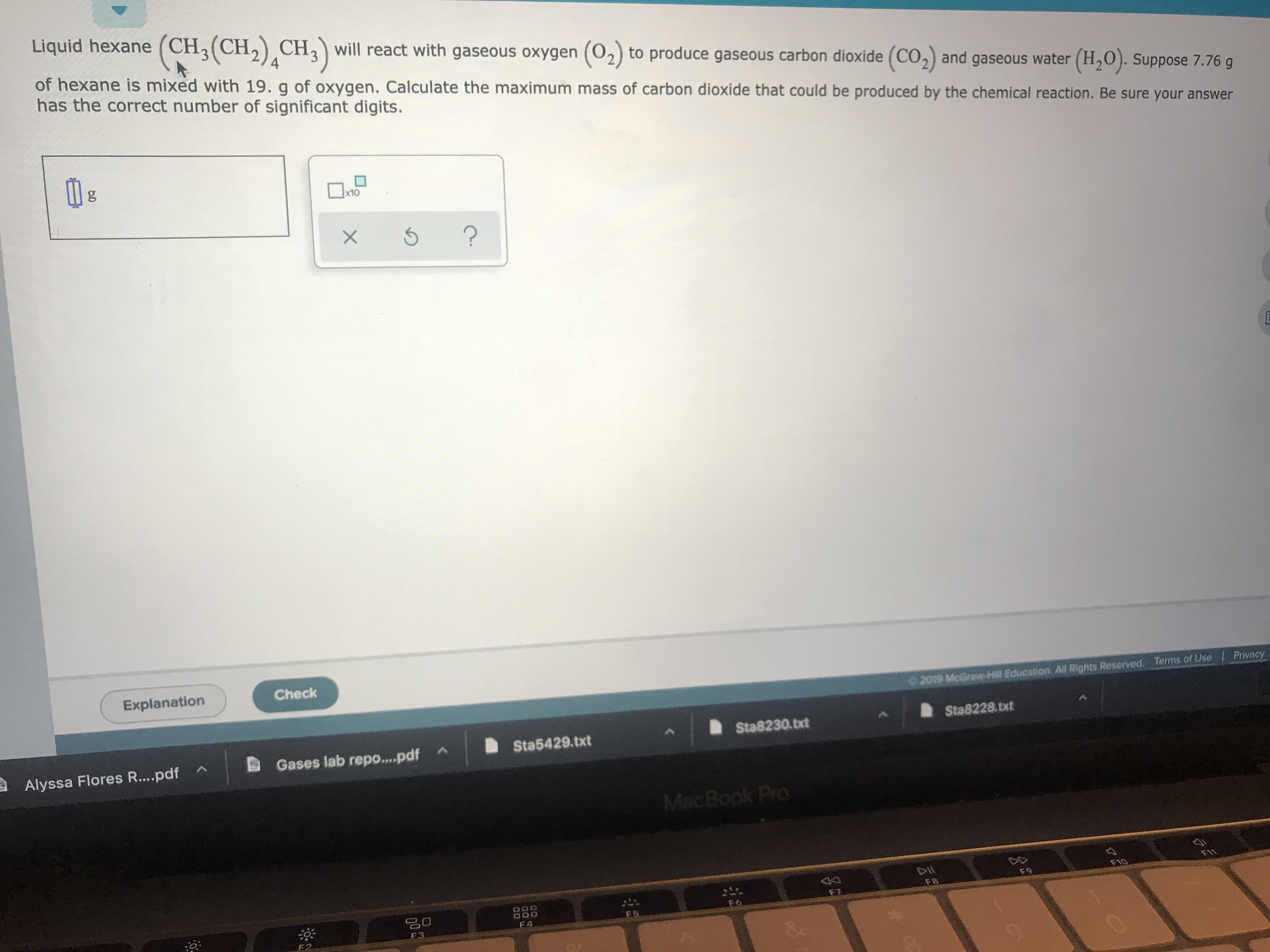
Transcribed Image Text:Liquid hexane (CH3(CH2) CH3will react with gaseous oxygen (02) to produce gaseous carbon dioxide (CO2) and gaseous water (H2O). Suppose 7.76 g
4
of hexane is mixed with 19. g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer
has the correct number of significant digits.
x10
?
Check
Explanation
Privacy
Terms of Use
2019 McGraw Hill Education All Rights Reserved.
Sta8228.txt
Sta8230.txt
Sta5429.txt
Gases lab repo....pdf
Alyssa Flores R....pdf
MacBook Pro
Dil
F 10
F9
F8
E7
F6
F5
F4
F3
F2
X
రు
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 5 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning