i. A brief explanation of how the nature of the ligand (strong-field or weak-field) will affect the magnetic moment of a d-block compound. Use a specific example of two different octahedral complexes containing the same metal ion and different ligands and the crystal field splitting diagrams to illustrate your point.
i. A brief explanation of how the nature of the ligand (strong-field or weak-field) will affect the magnetic moment of a d-block compound. Use a specific example of two different octahedral complexes containing the same metal ion and different ligands and the crystal field splitting diagrams to illustrate your point.
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.30QAP
Related questions
Question
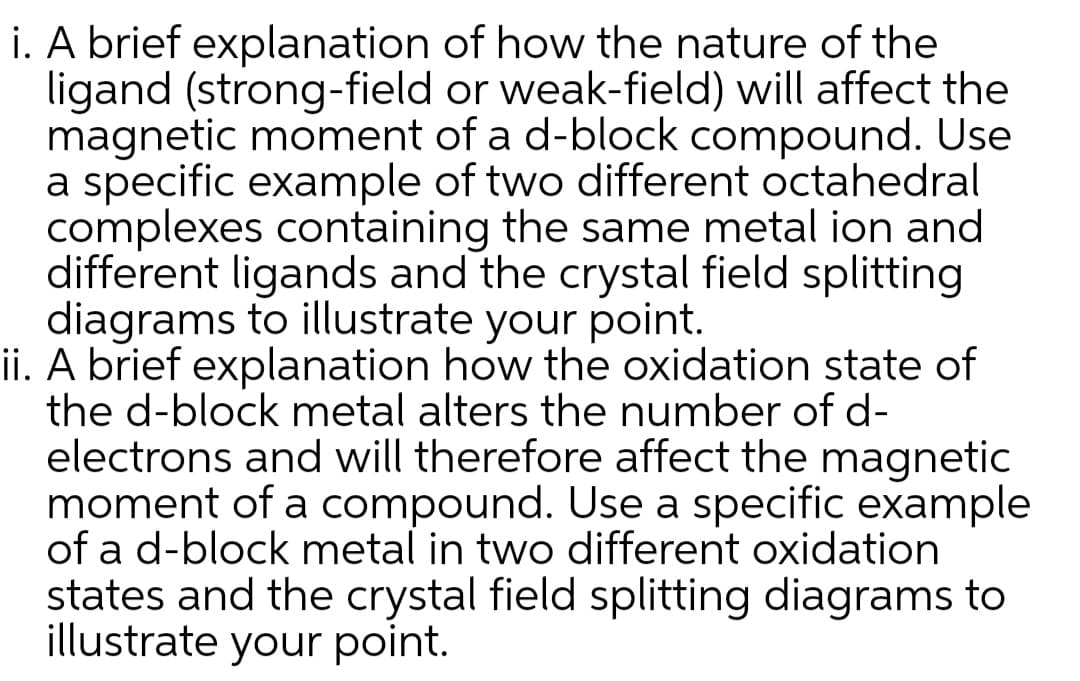
Transcribed Image Text:i. A brief explanation of how the nature of the
ligand (strong-field or weak-field) will affect the
magnetic moment of a d-block compound. Use
a specific example of two different octahedral
complexes containing the same metal ion and
different ligands and the crystal field splitting
diagrams to illustrate your point.
ii. A brief explanation how the oxidation state of
the d-block metal alters the number of d-
electrons and will therefore affect the magnetic
moment of a compound. Use a specific example
of a d-block metal in two different oxidation
states and the crystal field splitting diagrams to
illustrate your point.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning