I. Molecule's Degree of Unsaturation* *to avoid confusion, we shall use "degree of unsaturation" to refer to the number of pi bonds or number of rings present in the molecule as a result of the loss of hydrogen atoms when referenced using the alkane formula of C,„H2n+2" The numerical value for the degree of unsaturation = 1 means that a molecule of hydrogen, H,, or two atoms of hydrogen are lost and instead 1 pi bond OR 1 ring is seen in the molecule. For this exercise, determine the degree of unsaturation of each of the following compounds: CH3 1. 2,3,3-Trimethyl-1,4,6-octatriene CH3 6. CH3 2. 3-Ethyl-2,2,-dimethyl-3-heptene `CH3 3. 2-Methyl-1,5-hexadiene 8. 7. ÇH3 H. CH3 4. 4-tert-Butyl-2-methylheptane Answers: 1. L2. 3. _4. 5. 6. _7. 8. 5.
I. Molecule's Degree of Unsaturation* *to avoid confusion, we shall use "degree of unsaturation" to refer to the number of pi bonds or number of rings present in the molecule as a result of the loss of hydrogen atoms when referenced using the alkane formula of C,„H2n+2" The numerical value for the degree of unsaturation = 1 means that a molecule of hydrogen, H,, or two atoms of hydrogen are lost and instead 1 pi bond OR 1 ring is seen in the molecule. For this exercise, determine the degree of unsaturation of each of the following compounds: CH3 1. 2,3,3-Trimethyl-1,4,6-octatriene CH3 6. CH3 2. 3-Ethyl-2,2,-dimethyl-3-heptene `CH3 3. 2-Methyl-1,5-hexadiene 8. 7. ÇH3 H. CH3 4. 4-tert-Butyl-2-methylheptane Answers: 1. L2. 3. _4. 5. 6. _7. 8. 5.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL2: Mass Spectrometry
Section: Chapter Questions
Problem 20CTQ
Related questions
Question
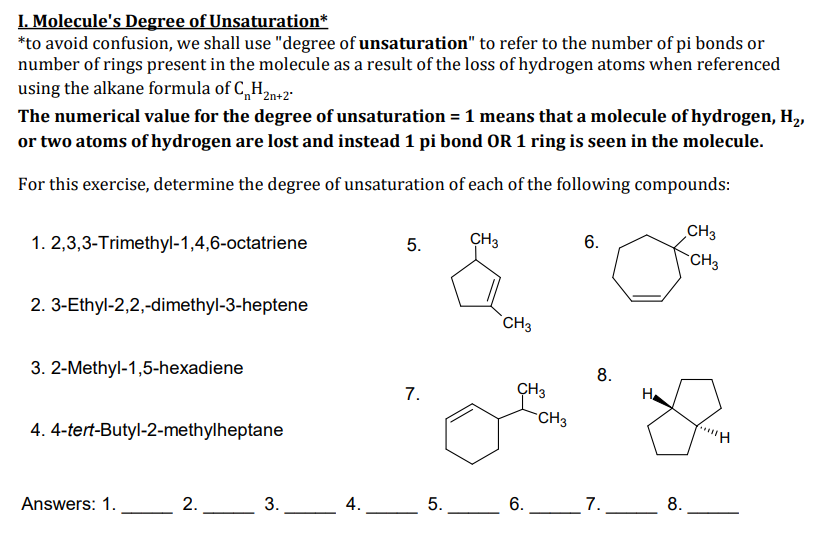
Transcribed Image Text:I. Molecule's Degree of Unsaturation*
*to avoid confusion, we shall use "degree of unsaturation" to refer to the number of pi bonds or
number of rings present in the molecule as a result of the loss of hydrogen atoms when referenced
using the alkane formula of C,„H2n+2"
The numerical value for the degree of unsaturation = 1 means that a molecule of hydrogen, H,,
or two atoms of hydrogen are lost and instead 1 pi bond OR 1 ring is seen in the molecule.
For this exercise, determine the degree of unsaturation of each of the following compounds:
CH3
1. 2,3,3-Trimethyl-1,4,6-octatriene
CH3
6.
CH3
2. 3-Ethyl-2,2,-dimethyl-3-heptene
`CH3
3. 2-Methyl-1,5-hexadiene
8.
7.
ÇH3
H.
CH3
4. 4-tert-Butyl-2-methylheptane
Answers: 1.
L2.
3.
_4.
5.
6.
_7.
8.
5.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning