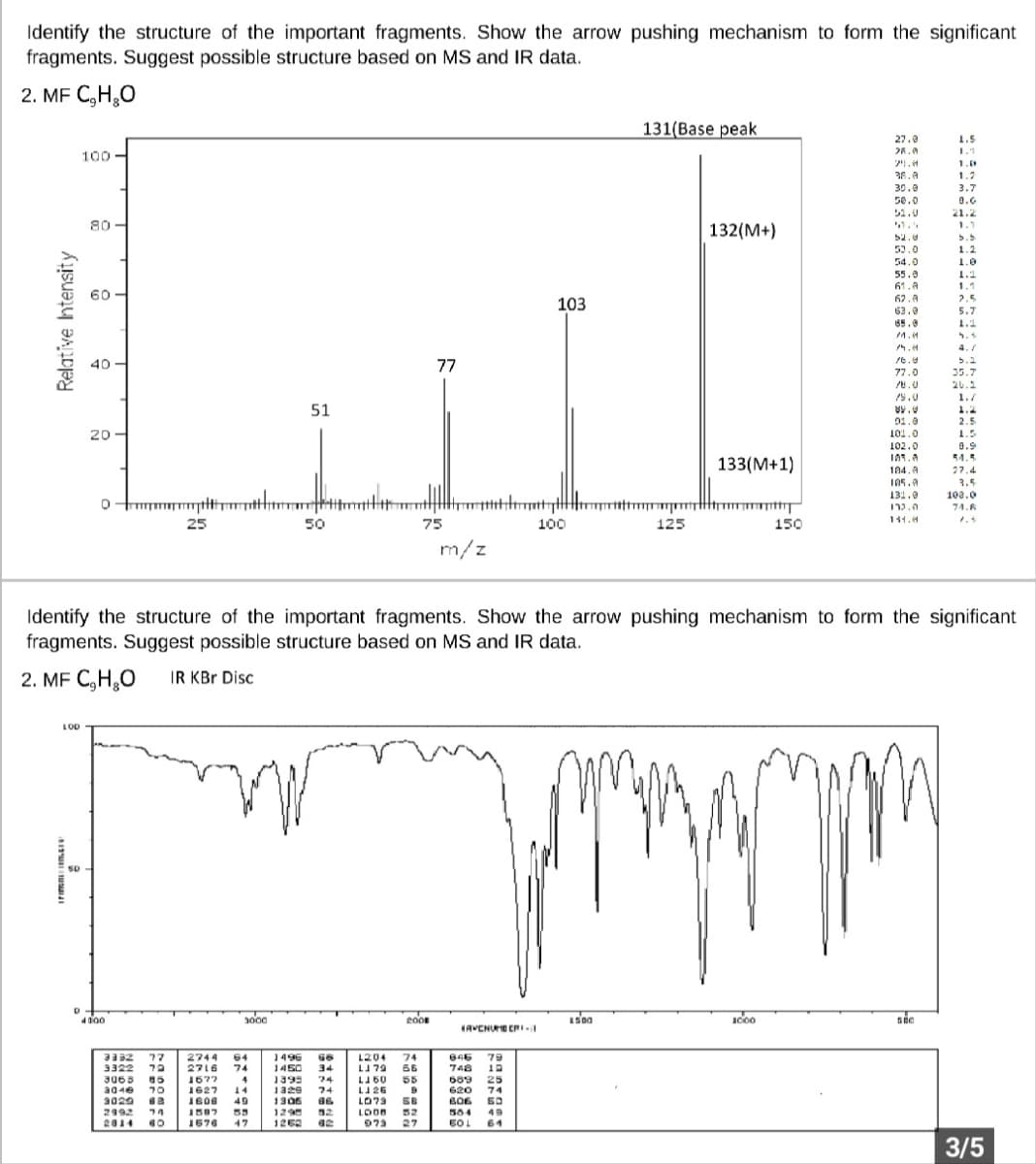
Identify the structure of the important fragments. Show the arrow pushing mechanism to form the significant fragments. Suggest possible structure based on MS and IR data. 2. MF C₂H₂O 131(Base peak 27.0 1.5 28.0 1.1 100 24.0 38.0 1.2 30.0 3.7 50.0 8.G 52.0 21.2 80 1.1 52.0 b.b 52.0 1.2 54.0 1.0 55.0 1.2 61.A 1.1 60- 62.A 2.5 63.0 5.7 65.0 1.2 M.M 40 16.0 5.2 77 77.0 35.7 78.0 20.1 79.0 1./ 51 ww. 1.2 01.0 2.5 20 101.0 1.5 102.0 0.9 101.0 54.5 104.9 27.4 105.0 3.5 131.0 100.0 h 132.0 74.6 25 141.4 50 100 125 150 m/z Identify the structure of the important fragments. Show the arrow pushing mechanism to form the significant fragments. Suggest possible structure based on MS and IR data. 2. MF C₂H₂O IR KBr Disc LOD n www 50 1500 1000 500 HAVENUE CRI Relative Intensity D 4100 3000 3332 77 2744 64 3322 79 2716 74 3063 85 1677 4 3040 70 1627 14 3029 63 1606 49 2992 74 1587 55 2014 60 1676 47 1496 66 1450 34 1395 74 1329 74 1306 66 1295 52 1262 62 75 2008 1204 74 LJ 79 66 L160 55 LJ 26 LO73 56 LOOD 52 973 27 9 totdat 846 79 748 10 689 25 620 74 606 60 564 49 60L 61 103 132(M+) 133(M+1) 3/5
Analyzing Infrared Spectra
The electromagnetic radiation or frequency is classified into radio-waves, micro-waves, infrared, visible, ultraviolet, X-rays and gamma rays. The infrared spectra emission refers to the portion between the visible and the microwave areas of electromagnetic spectrum. This spectral area is usually divided into three parts, near infrared (14,290 – 4000 cm-1), mid infrared (4000 – 400 cm-1), and far infrared (700 – 200 cm-1), respectively. The number set is the number of the wave (cm-1).
IR Spectrum Of Cyclohexanone
It is the analysis of the structure of cyclohexaone using IR data interpretation.
IR Spectrum Of Anisole
Interpretation of anisole using IR spectrum obtained from IR analysis.
IR Spectroscopy
Infrared (IR) or vibrational spectroscopy is a method used for analyzing the particle's vibratory transformations. This is one of the very popular spectroscopic approaches employed by inorganic as well as organic laboratories because it is helpful in evaluating and distinguishing the frameworks of the molecules. The infra-red spectroscopy process or procedure is carried out using a tool called an infrared spectrometer to obtain an infrared spectral (or spectrophotometer).
Step by step
Solved in 4 steps with 3 images









