If 173 mL of neon at 39.9 °C is allowed to expand to 422 mL, what must the new temperature be to maintain constant pressure? Express your answer in °C. You do not need to enter units.
If 173 mL of neon at 39.9 °C is allowed to expand to 422 mL, what must the new temperature be to maintain constant pressure? Express your answer in °C. You do not need to enter units.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.27QP
Related questions
Question
Question 6
Transcribed Image Text:A sandhills.mrooms3.net
Mall - Lafell.Sommer.095 - Outlook
Lafell Sommer
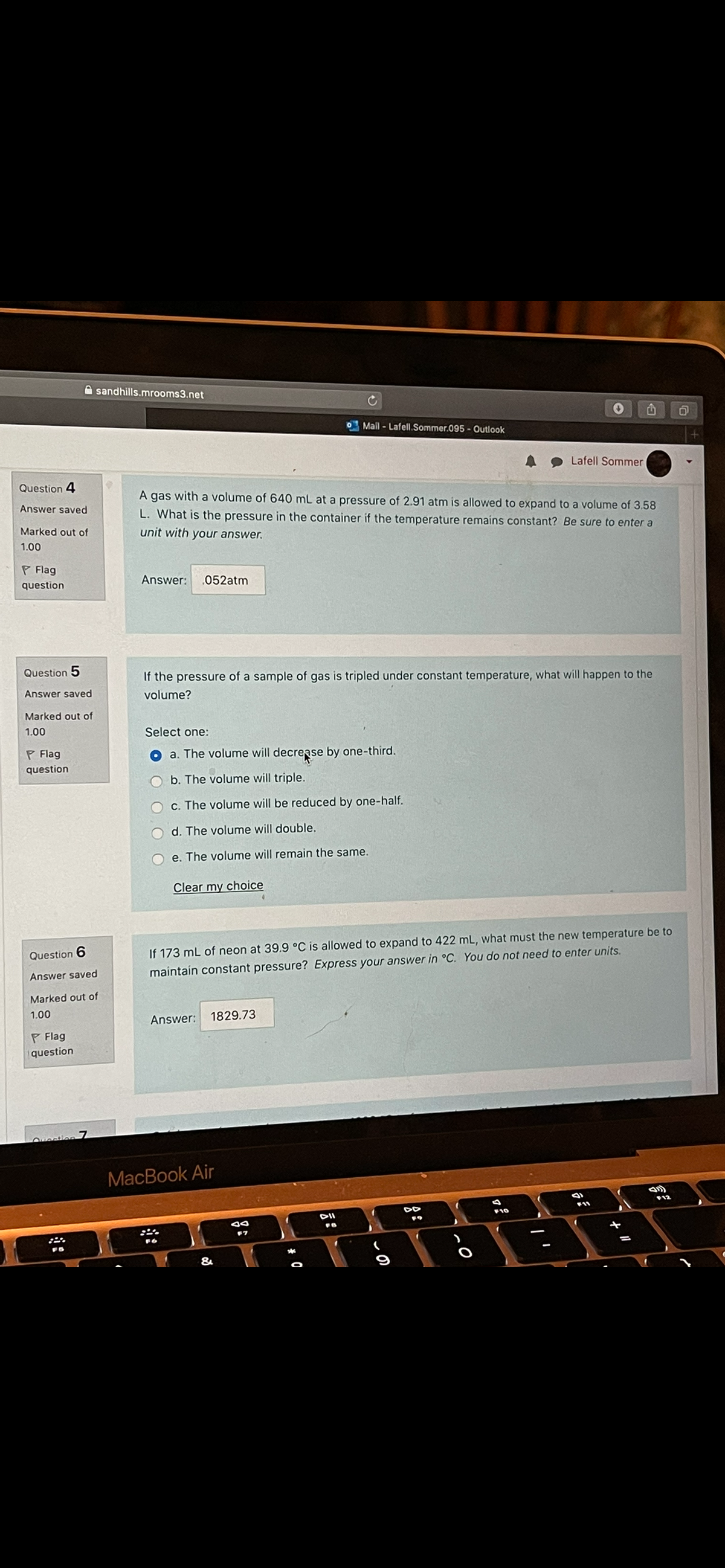
Question 4
A gas with a volume of 640 mL at a pressure of 2.91 atm is allowed to expand to a volume of 3.58
Answer saved
L. What is the pressure in the container if the temperature remains constant? Be sure to enter a
Marked out of
unit with your answer.
1.00
P Flag
Answer:
.052atm
question
Question 5
If the pressure of a sample of gas is tripled under constant temperature, what will happen to the
Answer saved
volume?
Marked out of
1.00
Select one:
P Flag
a. The volume will decrease by one-third.
question
b. The volume will triple.
c. The volume will be reduced by one-half.
d. The volume will double.
e. The volume will remain the same.
Clear my choice
Question 6
If 173 mL of neon at 39.9 °C is allowed to expand to 422 mL, what must the new temperature be to
maintain constant pressure? Express your answer in °C. You do not need to enter units.
Answer saved
Marked out of
1.00
Answer:
1829.73
P Flag
question
MacBook Air
10
DII
F7
&
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning