Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
If a large marshmallow has a volume of 2.50 in^3 and density of 0.242 g/cm^3, how kuch would it weigh in grams?
Transcribed Image Text:P Pearson Sign In
O Course
6 1-29 postLecture.pptx
O 1-25 PostLecture.pptx
HEM111-1-27_PostLecture.
com/course.html?courseld=16485573&0penVellumHMAC=5c6d2c3e101e04dad4cd1d6bf862ad58#10001
O Students Home
O Sign In - Wittenberg.
Word
HMail - Bryce I. Ander.
O Microsoft Office Ho.
O Wittenberg Logon
Tn Dashboard
= 7.87 g/cm
2670 cm
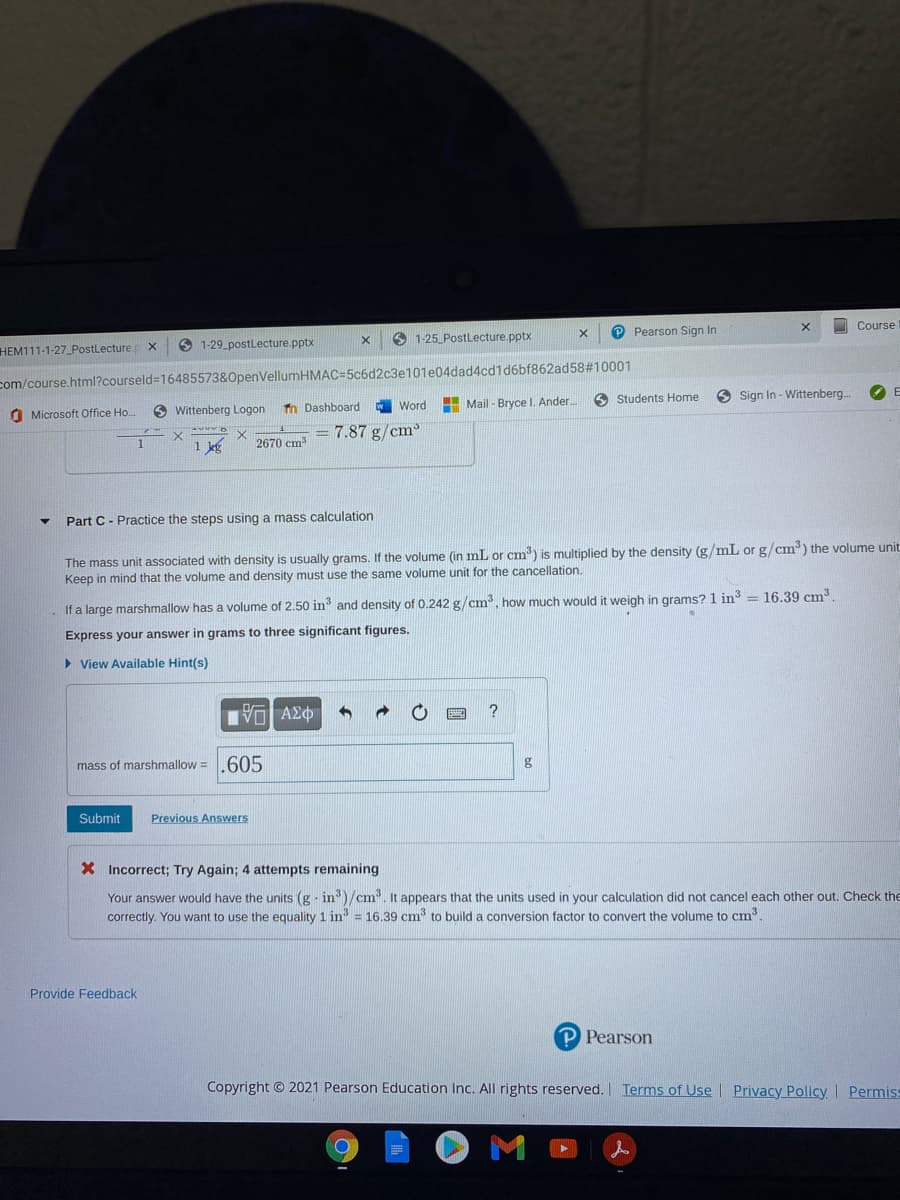
Part C- Practice the steps using a mass calculation
The mass unit associated with density is usually grams. If the volume (in mL or cm) is multiplied by the density (g/mL or g/cm) the volume unit
Keep in mind that the volume and density must use the same volume unit for the cancellation.
If a large marshmallow has a volume of 2.50 in and density of 0.242 g/cm³, how much would it weigh in grams? 1 in == 16.39 cm³
Express your answer in grams to three significant figures.
> View Available Hint(s)
να ΑΣφ
mass of marshmallow =605
Submit
Previous Answers
X Incorrect; Try Again; 4 attempts remaining
Your answer would have the units (g - in)/cm. It appears that the units used in your calculation did not cancel each other out. Check the
correctly. You want to use the equality 1 in = 16.39 cm to build a conversion factor to convert the volume to cm.
Provide Feedback
P Pearson
Copyright © 2021 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy | Permis:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning