If you had excess aluminum, how many moles of aluminum chloride could be produced from 26.0 g of chlorine gas, Cl2? Express your answer to three significant figures and include the appropriate units.
If you had excess aluminum, how many moles of aluminum chloride could be produced from 26.0 g of chlorine gas, Cl2? Express your answer to three significant figures and include the appropriate units.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 100CP: he production capacity for acrylonitrile (C3H3N)in the United States is over 2 billion pounds per...
Related questions
Question
100%
If you had excess aluminum, how many moles of aluminum chloride could be produced from 26.0 g of chlorine gas, Cl2?
Express your answer to three significant figures and include the appropriate units.
Transcribed Image Text:I Review I Constants I Periodic Table
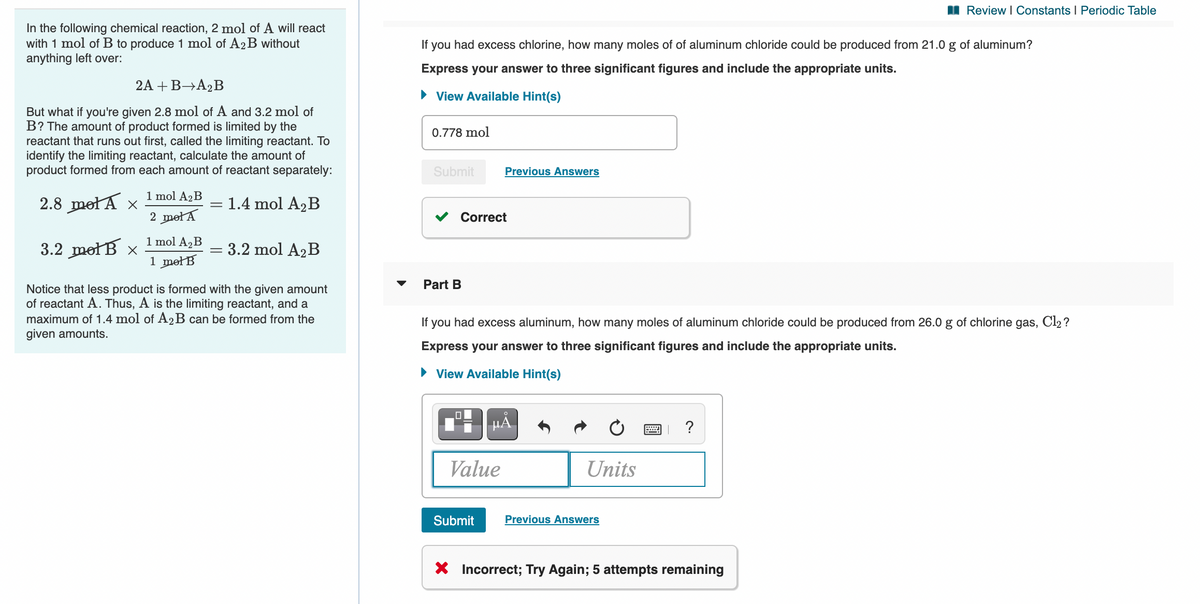
In the following chemical reaction, 2 mol of A will react
with 1 mol of B to produce 1 mol of A2B without
anything left over:
If
you
had excess chlorine, how many moles of of aluminum chloride could be produced from 21.0 g of aluminum?
Express your answer to three significant figures and include the appropriate units.
2A + B→A,B
• View Available Hint(s)
But what if you're given 2.8 mol of A and 3.2 mol of
B? The amount of product formed is limited by the
reactant that runs out first, called the limiting reactant. To
identify the limiting reactant, calculate the amount of
product formed from each amount of reactant separately:
0.778 mol
Submit
Previous Answers
1 mol A2B
2.8 metA x
1.4 mol A2B
2 metA
Correct
1 mol A,B
3.2 metB x
3.2 mol A2B
1 metB
Part B
Notice that less product is formed with the given amount
of reactant A. Thus, A is the limiting reactant, and a
maximum of 1.4 mol of A2B can be formed from the
given amounts.
If you had excess aluminum, how many moles of aluminum chloride could be produced from 26.0 g of chlorine gas, Cl2?
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
HA
?
Value
Units
Submit
Previous Answers
X Incorrect; Try Again; 5 attempts remaining
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div