Imagine that the cylinders in Figure 6 depict orbitals in another universe. Which statement about these orbitals is correct? Assume that you can apply the same scientific principles in in this universe than in our own. * a) The orbitals A and B are volumes around the nucleus where there is a high probability of finding an electron. b) An electron in orbital B will have more energy than an electron in orbital A. c) An atom will absorb energy when an electron makes a transition from orbital A to orbital B. d) All these statements about orbitals A and B are correct. e) None of these statements about orbitals A and B is correct
Imagine that the cylinders in Figure 6 depict orbitals in another universe. Which statement about these orbitals is correct? Assume that you can apply the same scientific principles in in this universe than in our own. * a) The orbitals A and B are volumes around the nucleus where there is a high probability of finding an electron. b) An electron in orbital B will have more energy than an electron in orbital A. c) An atom will absorb energy when an electron makes a transition from orbital A to orbital B. d) All these statements about orbitals A and B are correct. e) None of these statements about orbitals A and B is correct
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter22: Proteins
Section: Chapter Questions
Problem 22.50P: 22-50 Is a random coil a (a) primary, (b) secondary, (c) tertiary or (d) quaternary structure?...
Related questions
Question
Imagine that the cylinders in Figure 6 depict orbitals in another universe. Which statement about these orbitals is correct? Assume that you can apply the same scientific principles in in this universe than in our own. *
a) The orbitals A and B are volumes around the nucleus where there is a high probability of finding an electron.
b) An electron in orbital B will have more energy than an electron in orbital A.
c) An atom will absorb energy when an electron makes a transition from orbital A to orbital B.
d) All these statements about orbitals A and B are correct.
e) None of these statements about orbitals A and B is correct
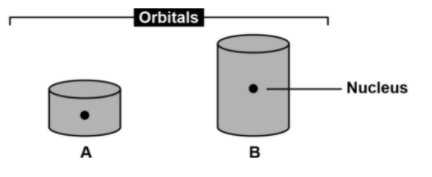
Transcribed Image Text:Orbitals
Nucleus
A
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning