In a synthesis of biodiesel, 8.7 g sunflower oil was used and 7.8 FAMEs was recovered. How much linoleic acid is generated using the cart below?
In a synthesis of biodiesel, 8.7 g sunflower oil was used and 7.8 FAMEs was recovered. How much linoleic acid is generated using the cart below?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section: Chapter Questions
Problem 45QRT
Related questions
Question
In a synthesis of biodiesel, 8.7 g sunflower oil was used and 7.8 FAMEs was recovered. How much linoleic acid is generated using the cart below?
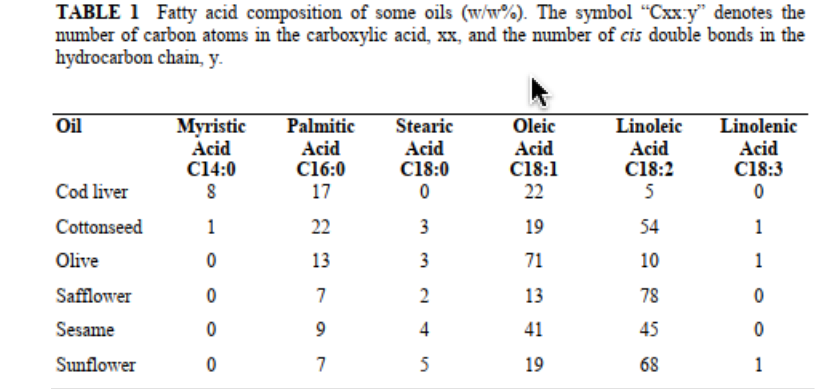
Transcribed Image Text:TABLE 1 Fatty acid composition of some oils (w/w%). The symbol "Cxx.y" denotes the
number of carbon atoms in the carboxylic acid, xx, and the number of cis double bonds in the
hydrocarbon chain, y.
Myristic
Acid
C14:0
Oil
Palmitic
Stearic
Oleic
Linoleic
Linolenic
Acid
C16:0
17
Acid
C18:0
Acid
C18:1
22
Acid
C18:2
Acid
C18:3
Cod liver
5
Cottonseed
1
22
3
19
54
1
Olive
13
3
71
10
1
Safflower
7
13
78
Sesame
4
41
45
Sunflower
7
5
19
68
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning