Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 89AP
Related questions
Question
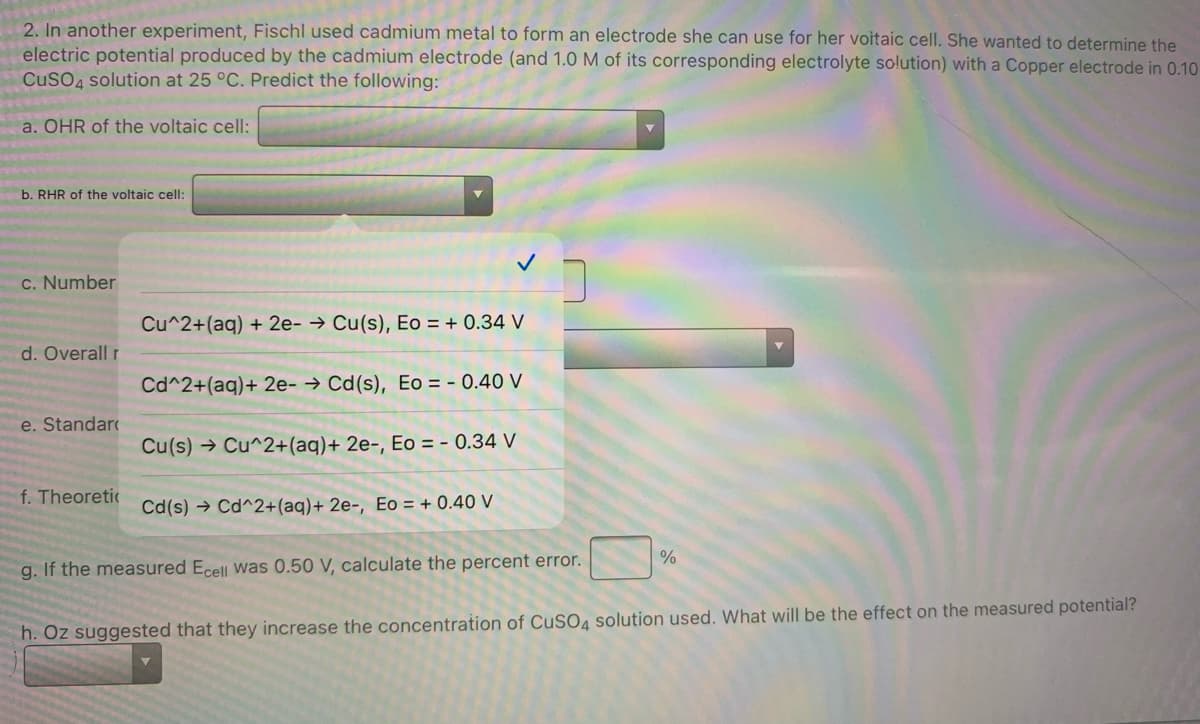
Transcribed Image Text:2. In another experiment, Fischl used cadmium metal to form an electrode she can use for her voitaic cell. She wanted to determine the
electric potential produced by the cadmium electrode (and 1.0 M of its corresponding electrolyte solution) with a Copper electrode in 0.10
CuSO4 solution at 25 °C. Predict the following:
a. OHR of the voltaic cell:
b. RHR of the voltaic cell:
c. Number
Cu^2+(ag) + 2e- → Cu(s), Eo = + 0.34 V
d. Overall r
Cd^2+(aq)+ 2e- → Cd(s), Eo = - 0.40 V
e. Standar
Cu(s) → Cu^2+(aq)+ 2e-, Eo = - 0.34 V
f. Theoretic
Cd(s) → Cd^2+(aq)+ 2e-, Eo = + 0.40 V
%
g. If the measured Ecell was 0.50 V, calculate the percent error.
h. Oz suggested that they increase the concentration of CuSO, solution used. What will be the effect on the measured potential?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning