General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.109QP: A 21.3-mL sample of 0.977 M NaOH is mixed with 29.5 mL of 0.918 M HCl in a coffee-cup calorimeter...
Related questions
Question

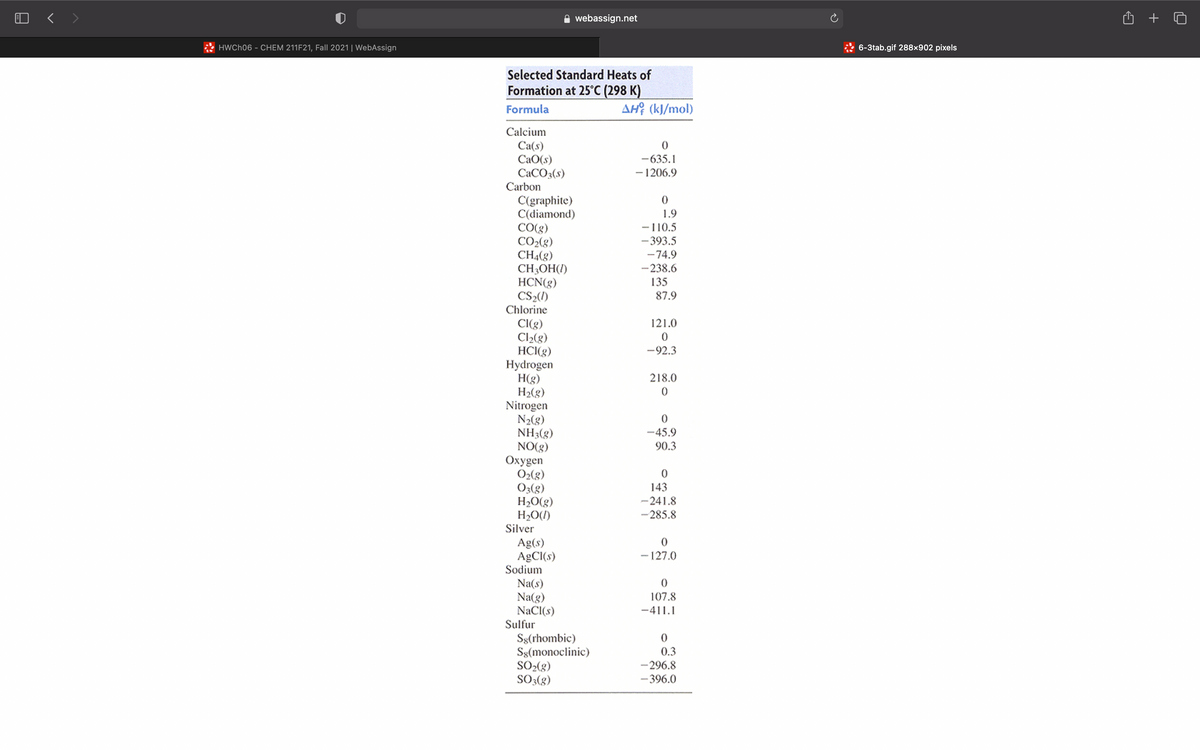
Transcribed Image Text:Use Table 6.3 or Appendix B to write balanced formation equations at standard conditions for each of the following compounds. (Type your answer using the format 03 for 03. Write all coefficients, even if they are fractions or 1.)
(a) (Apply fractions as needed.)
(s) +
(g)
AgCI(s)
(b) (Use the lowest possible coefficients.)
(s) +
(g) →
TIO2(s)
(c) (Apply fractions as needed.)
(g)
O3(9)
(d) (Apply fractions as needed.)
H2(g) +
(graphite) +
(g)
HCN(g)
Transcribed Image Text:O < >
webassign.net
+ O
HWCH06 - CHEM 211F21, Fall 2021| WebAssign
6-3tab.gif 288×902 pixels
Selected Standard Heats of
Formation at 25°C (298 K)
Formula
AH? (kJ/mol)
Calcium
Ca(s)
CaO(s)
CACO3(s)
Carbon
-635.1
- 1206.9
C(graphite)
C(diamond)
CO(g)
CO2(g)
CH4(8)
CH3OH(1)
HCN(g)
CS2()
1.9
-110.5
- 393.5
-74.9
-238.6
135
87.9
Chlorine
121.0
CI(g)
Cl2(g)
HCI(g)
Hydrogen
H(g)
H2(g)
Nitrogen
N2(g)
NH3(g)
NO(3)
Oxygen
O2(8)
O3(g)
H20(g)
H2O(1)
Silver
-92.3
218.0
-45.9
90.3
143
-241.8
- 285.8
Ag(s)
AGCI(s)
Sodium
- 127.0
Na(s)
Na(g)
NaCI(s)
Sulfur
107.8
-411.1
Sg(rhombic)
Ss(monoclinic)
SO-(g)
SO3(8)
0.3
- 296.8
- 396.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning