In chemistry class, a student conducted an investigation about factors affecting the solubility of copper (II) sulfate (CUSO4) in water. The data was recorded in the table below. Trial Factors Compound not stirred 1 Temperature: 60ºC Large crystals Compound was stirred 2 Temperature: 25°C Large crystals Compound was not stirred 3 Temperature 25°C Large crystals Compound was stirred 4 Temperature 60°C Finely crushed powder Which trial will have the slowest rate of dissolving? Explain the reasoning for your answer.
In chemistry class, a student conducted an investigation about factors affecting the solubility of copper (II) sulfate (CUSO4) in water. The data was recorded in the table below. Trial Factors Compound not stirred 1 Temperature: 60ºC Large crystals Compound was stirred 2 Temperature: 25°C Large crystals Compound was not stirred 3 Temperature 25°C Large crystals Compound was stirred 4 Temperature 60°C Finely crushed powder Which trial will have the slowest rate of dissolving? Explain the reasoning for your answer.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.87QE
Related questions
Question
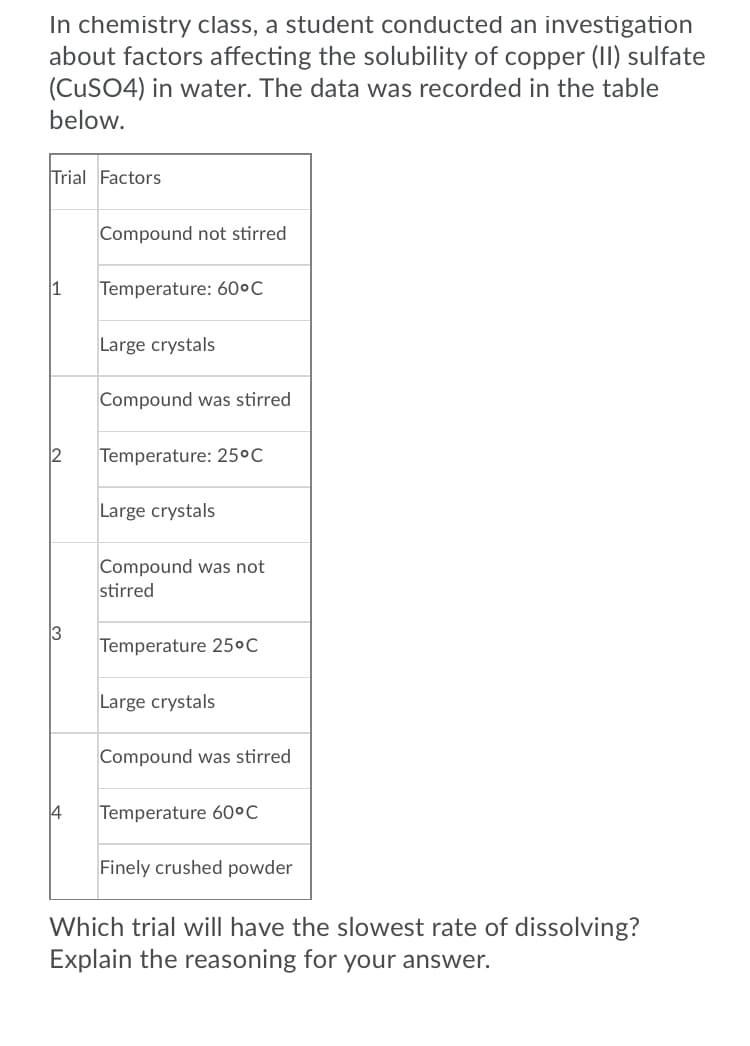
Transcribed Image Text:In chemistry class, a student conducted an investigation
about factors affecting the solubility of copper (II) sulfate
(CUSO4) in water. The data was recorded in the table
below.
Trial Factors
Compound not stirred
Temperature: 60ºC
Large crystals
Compound was stirred
2
Temperature: 25°C
Large crystals
Compound was not
stirred
3
Temperature 25°C
Large crystals
Compound was stirred
Temperature 60°C
Finely crushed powder
Which trial will have the slowest rate of dissolving?
Explain the reasoning for your answer.
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning