In the following table are listed the rate constants for the decomposition of N205 at different temperatures: t, °C k, sec 1 7.87 x 10-7 25 3.46 x 10-5 35 1.35 x 10 4 45 4.98 x 10-4 55 1.50 x 10 3 65 4.87 x 10 3 Determine the energy of activation and find the rate constant at 50°C. E, kJ/mol /sec *For the rate constant plegse use the format 1.00 x 10^-1
In the following table are listed the rate constants for the decomposition of N205 at different temperatures: t, °C k, sec 1 7.87 x 10-7 25 3.46 x 10-5 35 1.35 x 10 4 45 4.98 x 10-4 55 1.50 x 10 3 65 4.87 x 10 3 Determine the energy of activation and find the rate constant at 50°C. E, kJ/mol /sec *For the rate constant plegse use the format 1.00 x 10^-1
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.48PAE: 11.48 The following data were collected for the decomposition of NT),-: Time, f (min) [N2Os] (mol...
Related questions
Question
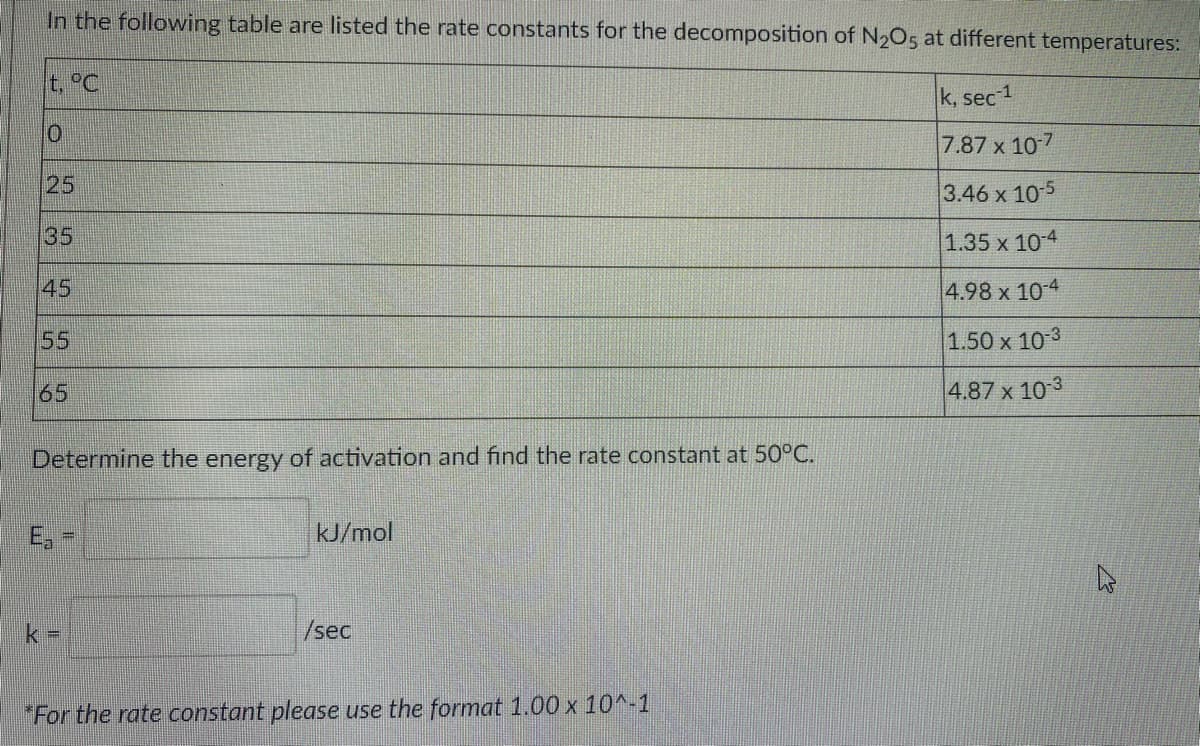
Transcribed Image Text:In the following table are listed the rate constants for the decomposition of N2O5 at different temperatures:
t, °C
k, sec 1
7.87 x 10-7
25
3.46 x 10-5
35
1.35 x 10-4
45
4.98 x 10-4
55
1.50 x 10 3
65
4.87 x 103
Determine the energy of activation and find the rate constant at 50°C.
E,
kJ/mol
/sec
"For the rate constant please use the format 1.00 x 10^-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning