In the synthesis of acetanilide, identify the data of the impure Sample: a. Mass of aniline: b. Mole of aniline: c. Theoretical moles of acetanilide: d. Theoretical mass of acetanilide:
In the synthesis of acetanilide, identify the data of the impure Sample: a. Mass of aniline: b. Mole of aniline: c. Theoretical moles of acetanilide: d. Theoretical mass of acetanilide:
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter17: Carboxylic Acids
Section: Chapter Questions
Problem 17.43P
Related questions
Question
100%
This the yt link of my professor gave me: https://www.youtube.com/watch?v=eFh9CfQltqo
In the synthesis of acetanilide, identify the data of the impure Sample:
a. Mass of aniline:
b. Mole of aniline:
c. Theoretical moles of acetanilide:
d. Theoretical mass of acetanilide:
(hi, this may be a simple question to you, but i no speak englis as my first language so pls bare w me. i am an us immigrant from thailand and still adjusting)
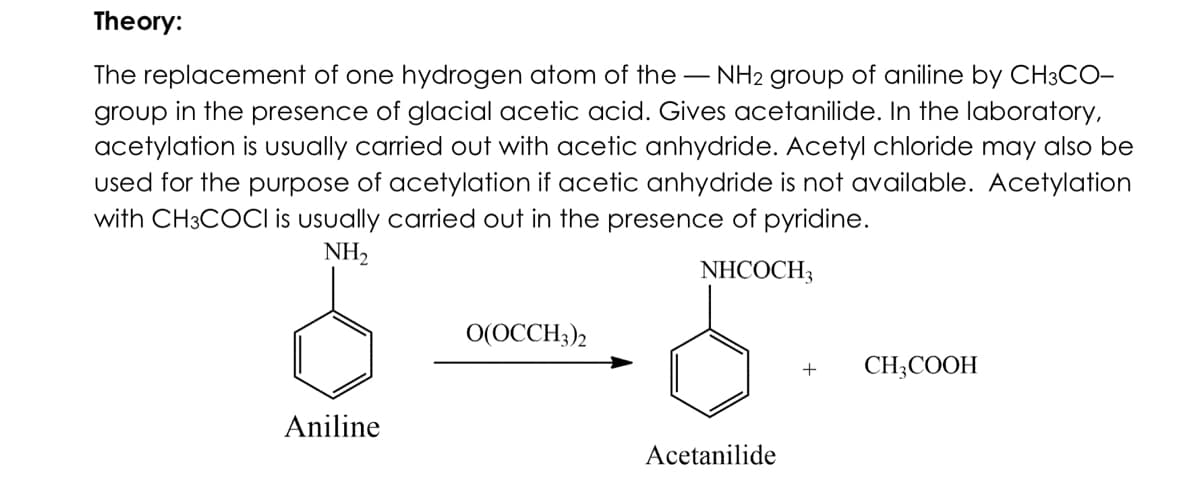
Transcribed Image Text:Theory:
The replacement of one hydrogen atom of the - NH2 group of aniline by CH3CO-
group in the presence of glacial acetic acid. Gives acetanilide. In the laboratory,
acetylation is usually carried out with acetic anhydride. Acetyl chloride may also be
used for the purpose of acetylation if acetic anhydride is not available. Acetylation
with CH3COCI is usually carried out in the presence of pyridine.
NH,
NHCOCH3
O(OCCH3)2
CH;COOH
Aniline
Acetanilide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
