In this question use the following equation to do the calculations in the questions below: ZnSO40) + HCly) → ZnCt2(m0) + H2SO4() a) For the calculation below, write the answer in scientific notation, round off correctly and make sure to use the correct significant figures. 5.97 + (1.9 x 136.9) b) If 3.89g of the ZnSO, will be used in the reaction, how many moles is being used? c) If 0.645mol of ZnCł2 needs to be made, how much of the starting HCt needs to be added in grams?
In this question use the following equation to do the calculations in the questions below: ZnSO40) + HCly) → ZnCt2(m0) + H2SO4() a) For the calculation below, write the answer in scientific notation, round off correctly and make sure to use the correct significant figures. 5.97 + (1.9 x 136.9) b) If 3.89g of the ZnSO, will be used in the reaction, how many moles is being used? c) If 0.645mol of ZnCł2 needs to be made, how much of the starting HCt needs to be added in grams?
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter1: Matter, Energy, And Measurement
Section: Chapter Questions
Problem 1.86P: 1-86 The specific heats of some elements at 25oC are as follows: aluminum = 0.215 cal/g · oC; carbon...
Related questions
Question
Q1
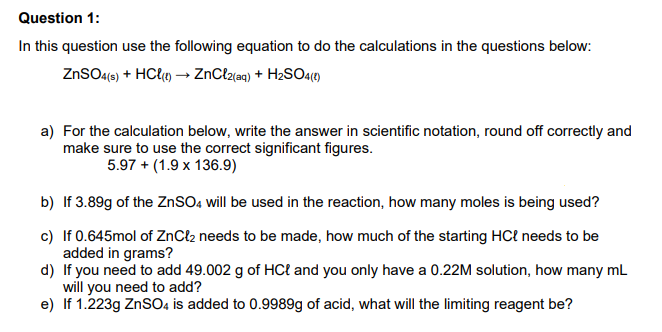
Transcribed Image Text:Question 1:
In this question use the following equation to do the calculations in the questions below:
ZNSO4(6) + HC{«) → ZnClz{aq) + H2SO4(1)
a) For the calculation below, write the answer in scientific notation, round off correctly and
make sure to use the correct significant figures.
5.97 + (1.9 x 136.9)
b) If 3.89g of the ZNSO, will be used in the reaction, how many moles is being used?
c) If 0.645mol of ZnCl2 needs to be made, how much of the starting HCł needs to be
added in grams?
d) If you need to add 49.002 g of HCł and you only have a 0.22M solution, how many mL
will you need to add?
e) If 1.223g ZnSO4 is added to 0.9989g of acid, what will the limiting reagent be?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning