Iron (II) ion is oxidized by chlorine in aqueous solution, the overall reaction being: 2Fe?+ (aq) + Cl2 (aq) → 2FE3+ (aq) + 2Cl- (aq) It is found experimentally that the rate of the overall reaction is decreased when either the iron (III) ion or the chloride ion concentration is increased. Is the following mechanism consistent with the experimental observations? Explain your answer in 2-3 complete sentences. Prove by finding the rate law for the proposed mechanism. Mechanism Step 1 Fe?+ (aq) + Cl2 (aq) = Fe4+ (aq) + 2Cl- (ag) (fast) Step 2 Fe+ (aq) + Fe2+ (aq) – > 2Fe3+ (ag) slow
Iron (II) ion is oxidized by chlorine in aqueous solution, the overall reaction being: 2Fe?+ (aq) + Cl2 (aq) → 2FE3+ (aq) + 2Cl- (aq) It is found experimentally that the rate of the overall reaction is decreased when either the iron (III) ion or the chloride ion concentration is increased. Is the following mechanism consistent with the experimental observations? Explain your answer in 2-3 complete sentences. Prove by finding the rate law for the proposed mechanism. Mechanism Step 1 Fe?+ (aq) + Cl2 (aq) = Fe4+ (aq) + 2Cl- (ag) (fast) Step 2 Fe+ (aq) + Fe2+ (aq) – > 2Fe3+ (ag) slow
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter18: Chemical Kinetics
Section: Chapter Questions
Problem 64AP
Related questions
Question
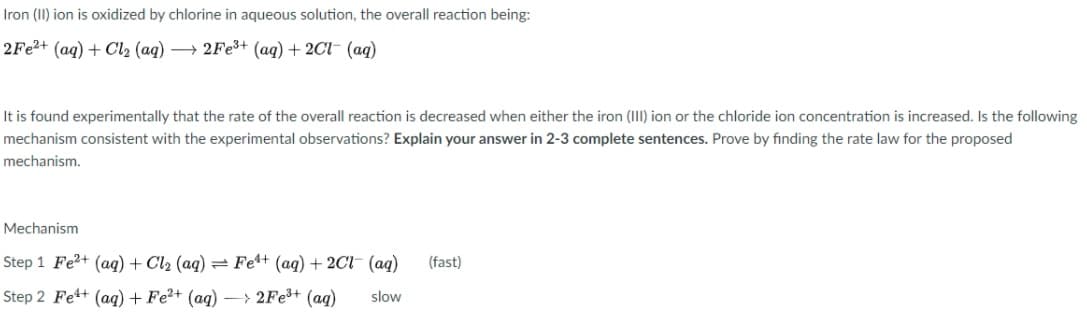
Transcribed Image Text:Iron (II) ion is oxidized by chlorine in aqueous solution, the overall reaction being:
2Fe?+ (aq) + Cl2 (aq) → 2FE3+ (aq) + 2Cl- (aq)
It is found experimentally that the rate of the overall reaction is decreased when either the iron (III) ion or the chloride ion concentration is increased. Is the following
mechanism consistent with the experimental observations? Explain your answer in 2-3 complete sentences. Prove by finding the rate law for the proposed
mechanism.
Mechanism
Step 1 Fe?+ (aq) + Cl2 (aq) = Fe4+ (aq) + 2Cl- (ag)
(fast)
Step 2 Fe+ (aq) + Fe2+ (aq) – > 2Fe3+ (ag)
slow
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax