is a description of the three-dimensional arrangement of atoms is a model used in chemistry to predict Complete the concepts below by supplying the appropriate term. within a molecule. The the geometry of individual molecules from the number of electron pai surrounding their central atoms. A molecule consisting of only two atoms has atoms bonded to the central shape. A molecule with unshared pair(s) of electrons has a linear shape atoms bonded to the central atom with atom with molecule with unshared pair(s) of electrons has a trigonal planar shape. The measure of the degree of inequality in the attraction of bonding electrons to various locations within a molecule is called A molecule may be when there is an equal sharing of electrons between the two atoms. While a molecule when it has a net dipole as a result of the opposing charges. may be
is a description of the three-dimensional arrangement of atoms is a model used in chemistry to predict Complete the concepts below by supplying the appropriate term. within a molecule. The the geometry of individual molecules from the number of electron pai surrounding their central atoms. A molecule consisting of only two atoms has atoms bonded to the central shape. A molecule with unshared pair(s) of electrons has a linear shape atoms bonded to the central atom with atom with molecule with unshared pair(s) of electrons has a trigonal planar shape. The measure of the degree of inequality in the attraction of bonding electrons to various locations within a molecule is called A molecule may be when there is an equal sharing of electrons between the two atoms. While a molecule when it has a net dipole as a result of the opposing charges. may be
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter4: Molecular Structure And Orbitals
Section: Chapter Questions
Problem 123CP: Carbon monoxide (CO) forms bonds to a variety of metals and metal ions. liS ability to bond to iron...
Related questions
Question
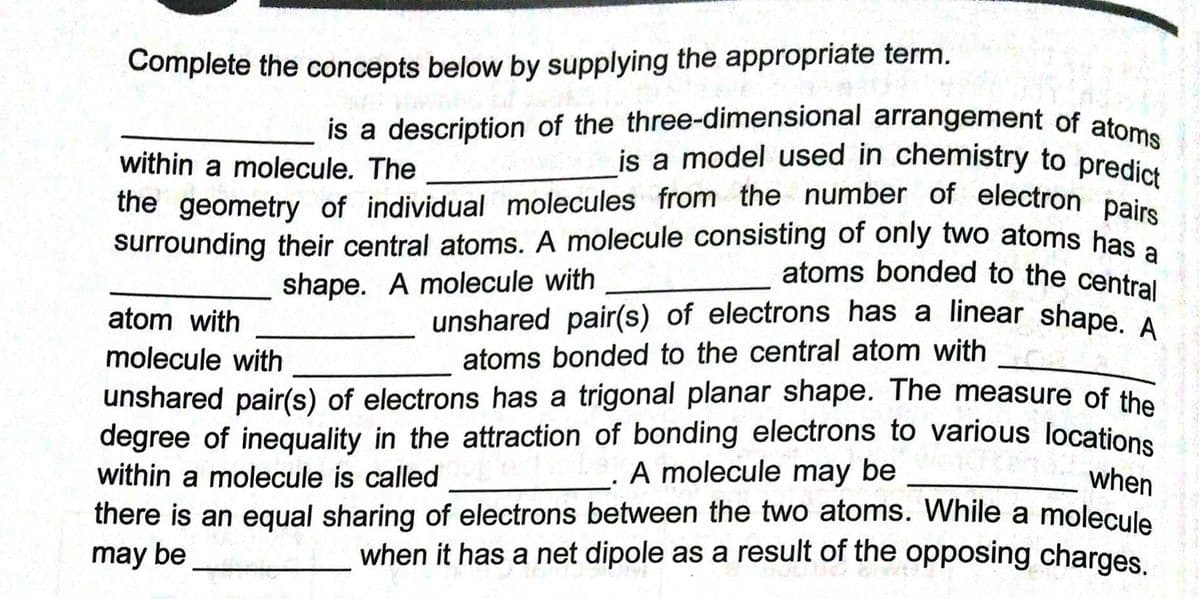
Transcribed Image Text:is a model used in chemistry to predict
the geometry of individual molecules from the number of electron pairs
unshared pair(s) of electrons has a linear shape. A
is a description of the three-dimensional arrangement of atoms
Complete the concepts below by supplying the appropriate term.
within a molecule. The
the geometry of individual molecules from the number of electron pe
surrounding their central atoms. A molecule consisting of only two atoms has
atoms bonded to the central
shape. A molecule with
atom with
molecule with
atoms bonded to the central atom with
unshared pair(s) of electrons has a trigonal planar shape. The measure of the
degree of inequality in the attraction of bonding electrons to various locations
within a molecule is called
there is an equal sharing of electrons between the two atoms. While a molecule
may be
A molecule may be
when
when it has a net dipole as a result of the opposing charges.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning