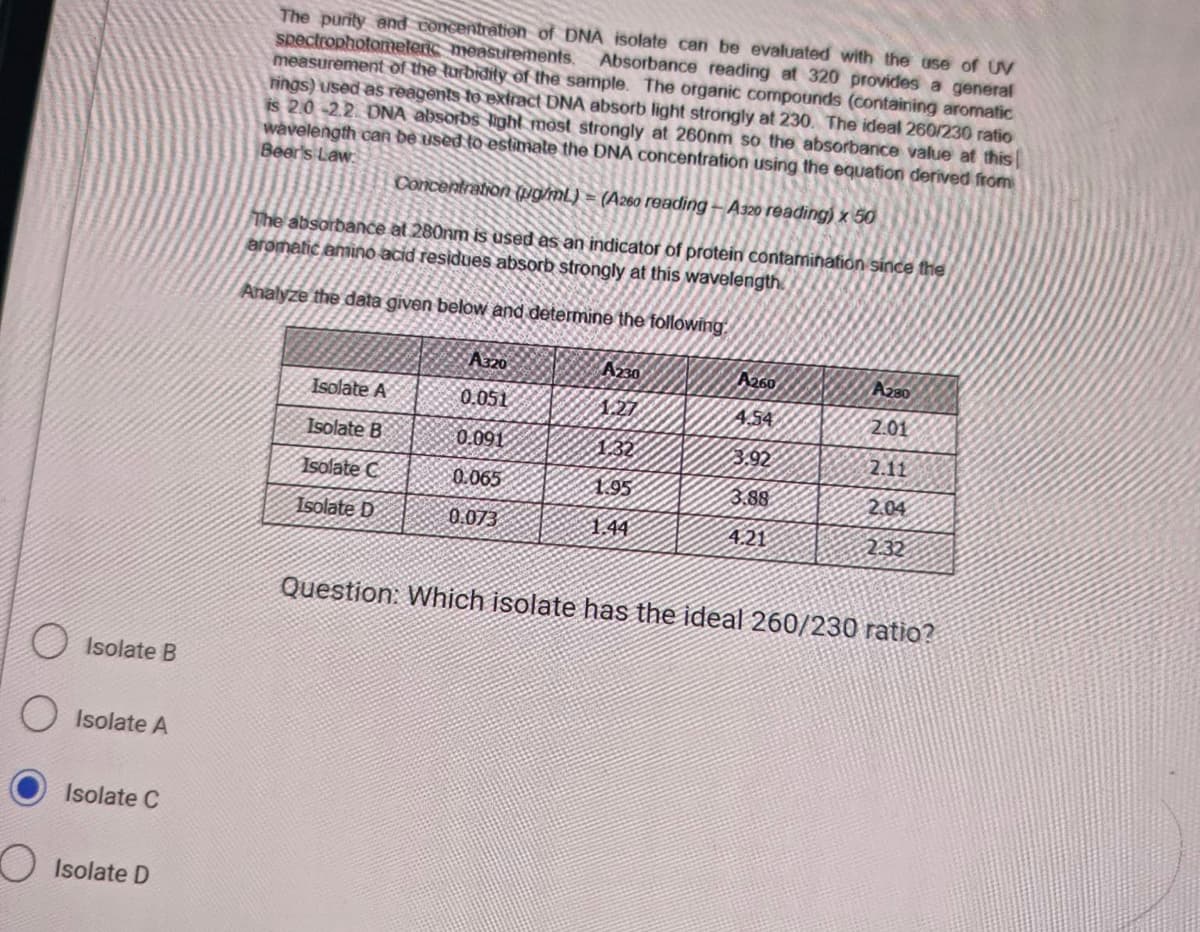
Isolate B Isolate A Isolate C Isolate D The purity and concentration of DNA isolate can be evaluated with the use of UV spectrophotomelenc measurements. measurement of the turbidity of the sample. The organic compounds (containing aromatic Absorbance reading at 320 provides a general hings) used as reagents to extract DNA absorb light strongly at 230. The ideal 260/230 ratio is 2.0-2.2. DNA absorbs light most strongly at 260nm so the absorbance value at this wavelength can be used to estimate the DNA concentration using the equation derived from Beer's Law Concentration (pg/mL) = (A260 reading-A320 reading) x 50 The absorbance at 280nm is used as an indicator of protein contamination since the aromatic amino acid residues absorb strongly at this wavelength Analyze the data given below and determine the following Isolate A Isolate B Isolate C Isolate D A320 0.051 0.091 0.065 0.073 A230 1.95 144 A260 4.54 3.92 3.88 4.21 A280 201 2.11 2.04 232 Question: Which isolate has the ideal 260/230 ratio?
Isolate B Isolate A Isolate C Isolate D The purity and concentration of DNA isolate can be evaluated with the use of UV spectrophotomelenc measurements. measurement of the turbidity of the sample. The organic compounds (containing aromatic Absorbance reading at 320 provides a general hings) used as reagents to extract DNA absorb light strongly at 230. The ideal 260/230 ratio is 2.0-2.2. DNA absorbs light most strongly at 260nm so the absorbance value at this wavelength can be used to estimate the DNA concentration using the equation derived from Beer's Law Concentration (pg/mL) = (A260 reading-A320 reading) x 50 The absorbance at 280nm is used as an indicator of protein contamination since the aromatic amino acid residues absorb strongly at this wavelength Analyze the data given below and determine the following Isolate A Isolate B Isolate C Isolate D A320 0.051 0.091 0.065 0.073 A230 1.95 144 A260 4.54 3.92 3.88 4.21 A280 201 2.11 2.04 232 Question: Which isolate has the ideal 260/230 ratio?
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
Transcribed Image Text:Isolate B
O Isolate A
Isolate C
O Isolate D
The purity and concentration of DNA isolate can be evaluated with the use of UV
spectrophotomeleric measurements.
measurement of the turbidity of the sample. The organic compounds (containing aromatic
Absorbance reading at 320 provides a general
rings) used as reagents to extract DNA absorb light strongly at 230. The ideal 260/230 ratio
is 2.0-2.2. DNA absorbs light most strongly at 260nm so the absorbance value at this
wavelength can be used to estimate the DNA concentration using the equation derived from
Beer's Law:
Concentration (pg/mL) = (A260 reading -A320 reading) x 50
The absorbance at 280nm is used as an indicator of protein contamination since the
aromatic amino acid residues absorb strongly at this wavelength
Analyze the data given below and determine the following.
Isolate A
Isolate B
Isolate C
Isolate D
A320
0.051
0.091
0.065
0.073
A230
1227
1.32
1.95
1.44
A260
4.54
3.92
3.88
4.21
A280
2.01
2.11
2.04
2.32
Question: Which isolate has the ideal 260/230 ratio?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON