It is desired to prepare polyester with the number average molecular weight, Mn 5000 g/mol by reacting of 1 mol butane-1,4 diol (HO-CH2-CH2-CH2-CH2-OH) with 1 mol of adipic acid (HOOC-CH2-CH2-CH2-CH2-COOH). (a) Write chemical equation illustrating synthesis of the polyester. (b) Calculate the value of the monomer conversion at which the reaction should be stopped to obtain this polymer, assuming perfect stoichiometric balance. What would be polydispersity index for the polymer obtained? (c) Assuming that 0.5 mol % of the diol is lost to polymerization by a side reaction (dehydratation to a non-reactive substance), what would be the value of Mn if the reaction was carried out to the same extent as in part (b). (d) Font
It is desired to prepare polyester with the number average molecular weight, Mn 5000 g/mol by reacting of 1 mol butane-1,4 diol (HO-CH2-CH2-CH2-CH2-OH) with 1 mol of adipic acid (HOOC-CH2-CH2-CH2-CH2-COOH). (a) Write chemical equation illustrating synthesis of the polyester. (b) Calculate the value of the monomer conversion at which the reaction should be stopped to obtain this polymer, assuming perfect stoichiometric balance. What would be polydispersity index for the polymer obtained? (c) Assuming that 0.5 mol % of the diol is lost to polymerization by a side reaction (dehydratation to a non-reactive substance), what would be the value of Mn if the reaction was carried out to the same extent as in part (b). (d) Font
Chapter32: Gas Chromatography
Section: Chapter Questions
Problem 32.23QAP
Related questions
Question
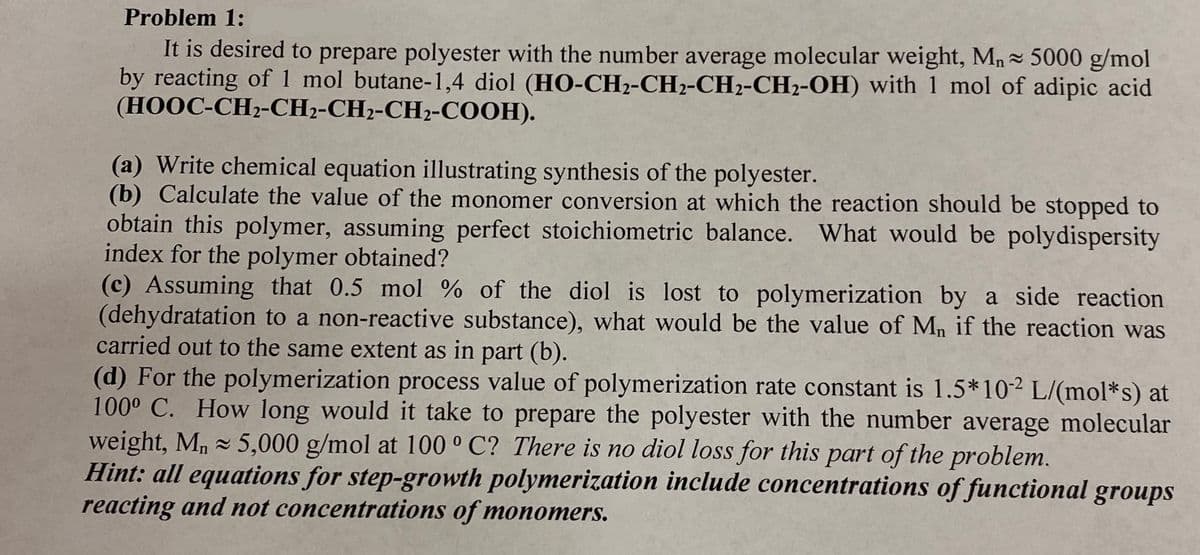
Transcribed Image Text:Problem 1:
It is desired to prepare polyester with the number average molecular weight, Mn- 5000 g/mol
by reacting of 1 mol butane-1,4 diol (HO-CH2-CH2-CH2-CH2-OH) with 1 mol of adipic acid
(HOOC-CH2-CH2-CH2-CH2-COOH).
(a) Write chemical equation illustrating synthesis of the polyester.
(b) Calculate the value of the monomer conversion at which the reaction should be stopped to
obtain this polymer, assuming perfect stoichiometric balance. What would be polydispersity
index for the polymer obtained?
(c) Assuming that 0.5 mol % of the diol is lost to polymerization by a side reaction
(dehydratation to a non-reactive substance), what would be the value of Mn if the reaction was
carried out to the same extent as in part (b).
(d) For the polymerization process value of polymerization rate constant is 1.5*102 L/(mol*s) at
100° C. How long would it take to prepare the polyester with the number average molecular
weight, M, 5,000 g/mol at 100 ° C? There is no diol loss for this part of the problem.
Hint: all equations for step-growth polymerization include concentrations of functional groups
reacting and not concentrations of monomers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning