Jessica is doing her pre-lab homework for Chemistry. She is asked to predict how many grams of oxygen gas are produced if 40.0 g of potassium chlorate is decomposed according to this balanced chemical equation shown. 2KCIO3(s)→2KCI(s)+302(g) She performs the following calculation, and gets 15.7 g of O2 as her answer. g KCIO, mol KCIO3 mol 02 g O2 1 mol KC103 122.55 g KC1O3 3 mol 02 2 mol KC103 32.00 g 02 1 тol 0z The next day when her group performs the experiment they obtain an actual yield of 15.3 grams of oxygen gas. What is the percent yield for the experiment? 'report your answer to the tenths place, i.e. one decimal place.
Jessica is doing her pre-lab homework for Chemistry. She is asked to predict how many grams of oxygen gas are produced if 40.0 g of potassium chlorate is decomposed according to this balanced chemical equation shown. 2KCIO3(s)→2KCI(s)+302(g) She performs the following calculation, and gets 15.7 g of O2 as her answer. g KCIO, mol KCIO3 mol 02 g O2 1 mol KC103 122.55 g KC1O3 3 mol 02 2 mol KC103 32.00 g 02 1 тol 0z The next day when her group performs the experiment they obtain an actual yield of 15.3 grams of oxygen gas. What is the percent yield for the experiment? 'report your answer to the tenths place, i.e. one decimal place.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 67QAP: When potassium chlorate is subjected to high temperatures, it decomposes into potassium chloride and...
Related questions
Question
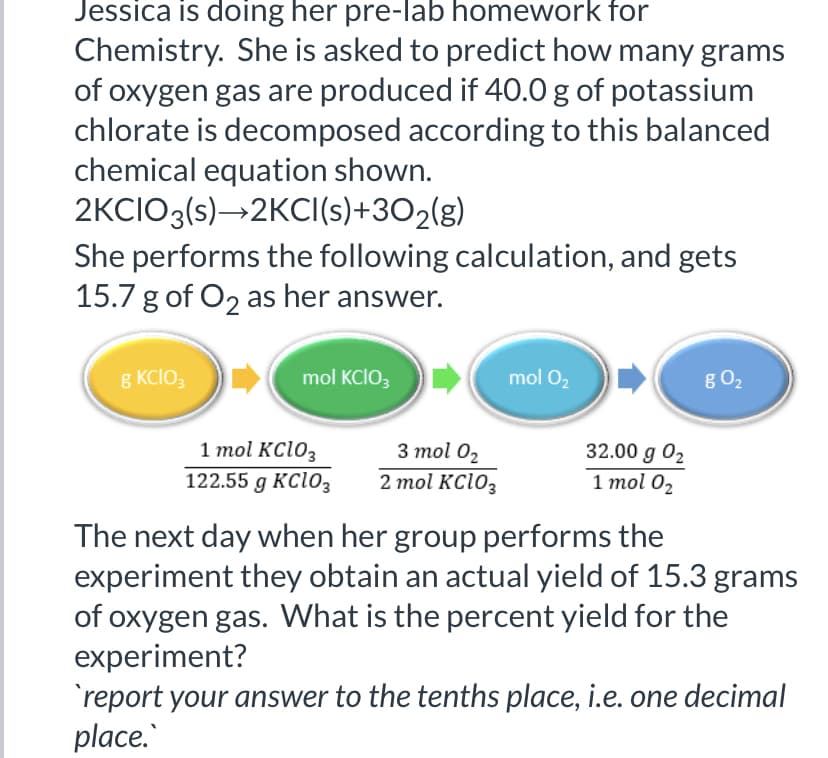
Transcribed Image Text:Jessica is doing her pre-lab homework for
Chemistry. She is asked to predict how many grams
of oxygen gas are produced if 40.0 g of potassium
chlorate is decomposed according to this balanced
chemical equation shown.
2KCIO3(s)→2KCI(s)+3O2(g)
She performs the following calculation, and gets
15.7 g of O2 as her answer.
8 KCIO,
mol KCIO;
mol O2
g O2
1 mol KC103
122.55 g KClo3
3 mol 02
32.00 g 02
1 mol 02
2 mol KC1O3
The next day when her group performs the
experiment they obtain an actual yield of 15.3 grams
of oxygen gas. What is the percent yield for the
experiment?
'report your answer to the tenths place, i.e. one decimal
place.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning