L Give Up? Hint Che Resources 2177/3100 e: At The molecule 2-butene is able to undergo a process called cis-trans isomerization, where the molecule switches from being a cis-alkene to a trans-alkene. This transformation can be induced by light. H H3C H H light C=C C=C CH3 CH3 H3C What is the hybridization of the two central carbon atoms in 2-butene? sp sp sp3 The isomerization requires breaking the t bond. Use the table of bond energies to determine the approximate amount of energy (in joules) required to break the C-C t bond in 2-butene, both per mole and per molecule. J/mol E: E = J/molecule What is the wavelength of light that would be required to perform the cis-trans isomerization of one molecule of 2-butene? nm Which region of the electromagnetic spectrum is this wavelength of light within? infrared ultaviolet SAMSUNG @wwige $ % & 4 5 6 7 t r i u y O f k j b V 00 re: 2177/3100 Lx Give Up? Resources light C=C C=C H3C CH3 CH3 H What is the hybridization of the two central carbon atoms in 2-butene? sp2 sp sp3 The isomerization requires breaking the t bond. Use the table of bond energies to determine the approximate ar energy (in joules) required to break the C-Cr bond in 2-butene, both mole and per molecule. per E = E = What is the wavelength of light that would be required to perform the cis-trans isomerization of one molecule of Which region of the electromagnetic spectrum is this wavelength of light within? infrared ultaviolet X-ray visible SAMSUNG $ & 4 6
L Give Up? Hint Che Resources 2177/3100 e: At The molecule 2-butene is able to undergo a process called cis-trans isomerization, where the molecule switches from being a cis-alkene to a trans-alkene. This transformation can be induced by light. H H3C H H light C=C C=C CH3 CH3 H3C What is the hybridization of the two central carbon atoms in 2-butene? sp sp sp3 The isomerization requires breaking the t bond. Use the table of bond energies to determine the approximate amount of energy (in joules) required to break the C-C t bond in 2-butene, both per mole and per molecule. J/mol E: E = J/molecule What is the wavelength of light that would be required to perform the cis-trans isomerization of one molecule of 2-butene? nm Which region of the electromagnetic spectrum is this wavelength of light within? infrared ultaviolet SAMSUNG @wwige $ % & 4 5 6 7 t r i u y O f k j b V 00 re: 2177/3100 Lx Give Up? Resources light C=C C=C H3C CH3 CH3 H What is the hybridization of the two central carbon atoms in 2-butene? sp2 sp sp3 The isomerization requires breaking the t bond. Use the table of bond energies to determine the approximate ar energy (in joules) required to break the C-Cr bond in 2-butene, both mole and per molecule. per E = E = What is the wavelength of light that would be required to perform the cis-trans isomerization of one molecule of Which region of the electromagnetic spectrum is this wavelength of light within? infrared ultaviolet X-ray visible SAMSUNG $ & 4 6
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.99QE: The molecular orbital diagram of NO shown in Figure 10.47 also applies to the following species....
Related questions
Question
Transcribed Image Text:L Give Up?
Hint
Che
Resources
2177/3100
e:
At
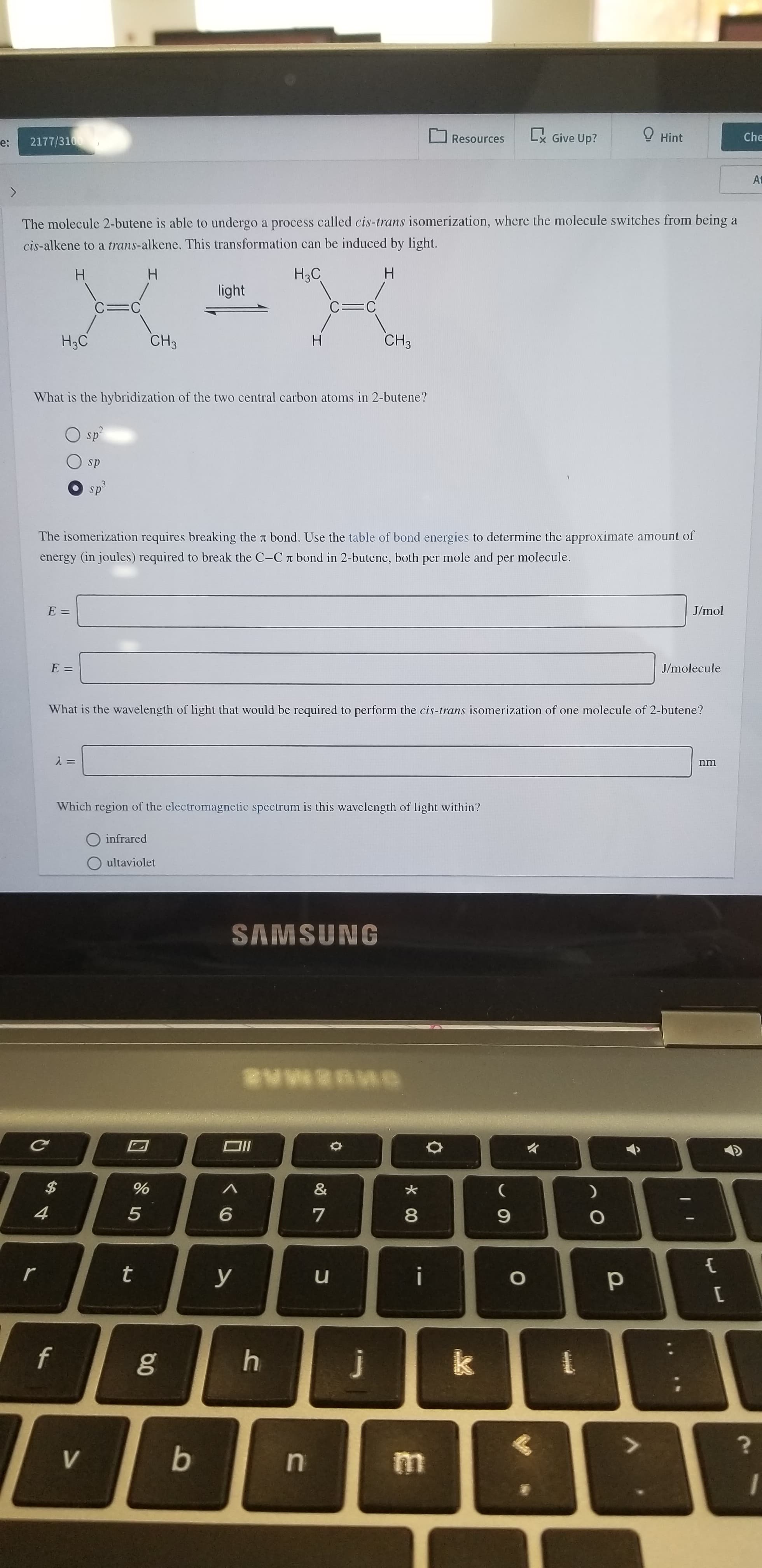
The molecule 2-butene is able to undergo a process called cis-trans isomerization, where the molecule switches from being a
cis-alkene to a trans-alkene. This transformation can be induced by light.
H
H3C
H
H
light
C=C
C=C
CH3
CH3
H3C
What is the hybridization of the two central carbon atoms in 2-butene?
sp
sp
sp3
The isomerization requires breaking the t bond. Use the table of bond energies to determine the approximate amount of
energy (in joules) required to break the C-C t bond in 2-butene, both per mole and per molecule.
J/mol
E:
E =
J/molecule
What is the wavelength of light that would be required to perform the cis-trans isomerization of one molecule of 2-butene?
nm
Which region of the electromagnetic spectrum is this wavelength of light within?
infrared
ultaviolet
SAMSUNG
@wwige
$
%
&
4
5
6
7
t
r
i
u
y
O
f
k
j
b
V
00
Transcribed Image Text:re:
2177/3100
Lx Give Up?
Resources
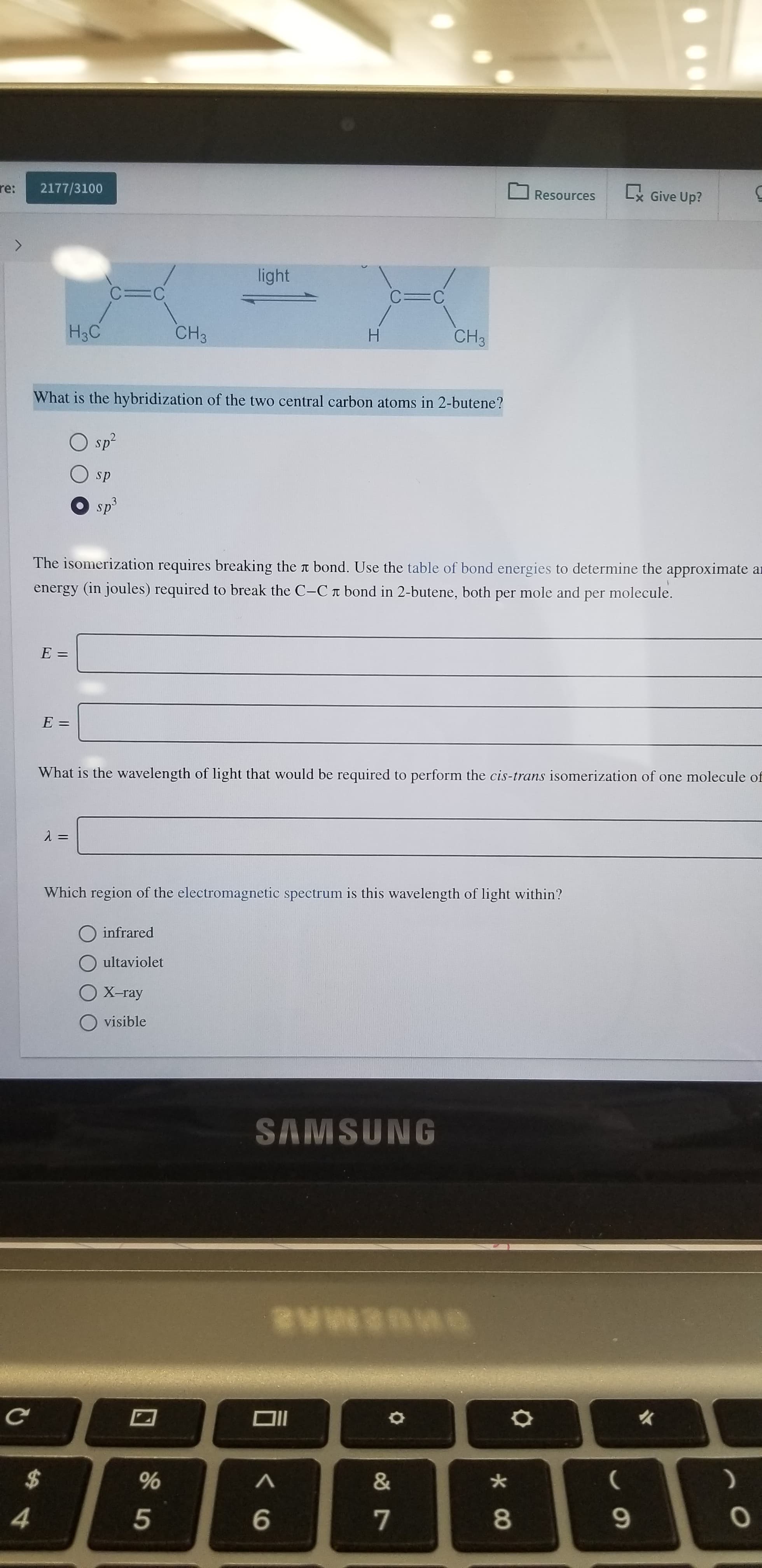
light
C=C
C=C
H3C
CH3
CH3
H
What is the hybridization of the two central carbon atoms in 2-butene?
sp2
sp
sp3
The isomerization requires breaking the t bond. Use the table of bond energies to determine the approximate ar
energy (in joules) required to break the C-Cr bond in 2-butene, both
mole and per molecule.
per
E =
E =
What is the wavelength of light that would be required to perform the cis-trans isomerization of one molecule of
Which region of the electromagnetic spectrum is this wavelength of light within?
infrared
ultaviolet
X-ray
visible
SAMSUNG
$
&
4
6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning