5) For the following reaction: I2 (g) + H2 (g) → 2HI (g) If the amount consumed from (I2) according to the above reaction is (50.8g) and the actual yield of HI is (40g) then the percentage yield of (HI) is equal to : a) 78.125% b) 15.625% c) 63.341% d)39.06% . 6) The remaining mass of (92g) of HNO3 if reacted with (24g) of LIOH according to the equation: ww w HNO3 + LIOH → LINO3 + H2 O a) 46 b) 29 c) 2 d) 11
5) For the following reaction: I2 (g) + H2 (g) → 2HI (g) If the amount consumed from (I2) according to the above reaction is (50.8g) and the actual yield of HI is (40g) then the percentage yield of (HI) is equal to : a) 78.125% b) 15.625% c) 63.341% d)39.06% . 6) The remaining mass of (92g) of HNO3 if reacted with (24g) of LIOH according to the equation: ww w HNO3 + LIOH → LINO3 + H2 O a) 46 b) 29 c) 2 d) 11
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.19PAE: 4.19 How many metric tons of carbon are required to react with 7.83 metric tons of Fe2O3 according...
Related questions
Question
100%
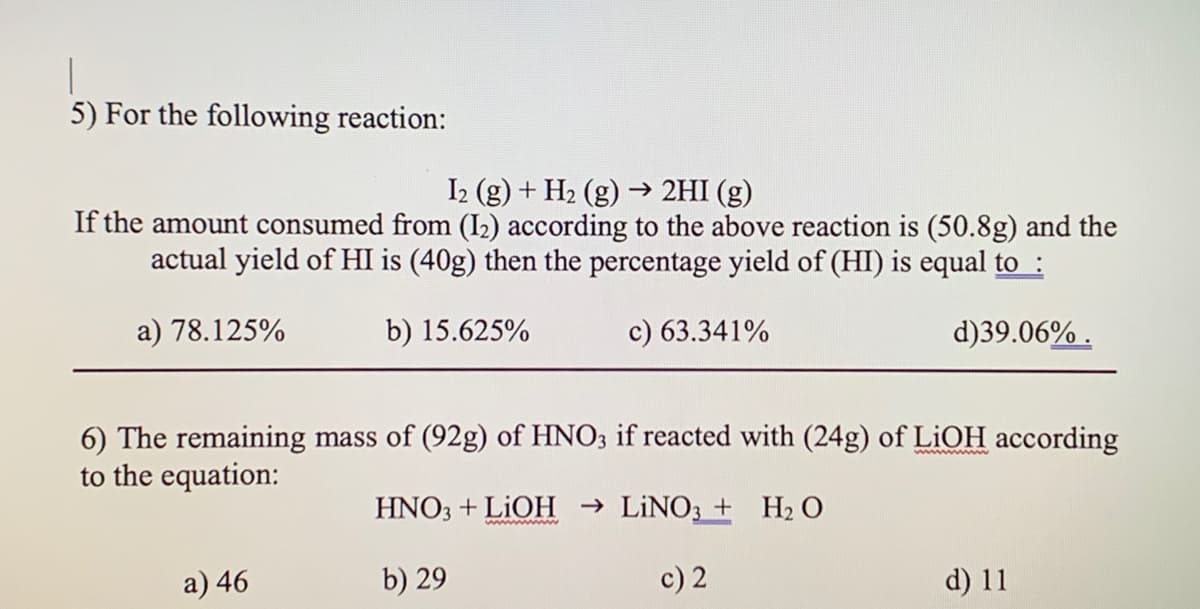
Transcribed Image Text:5) For the following reaction:
I2 (g) + H2 (g) → 2HI (g)
If the amount consumed from (I2) according to the above reaction is (50.8g) and the
actual yield of HI is (40g) then the percentage yield of (HI) is equal to :
a) 78.125%
b) 15.625%
c) 63.341%
d)39.06% .
6) The remaining mass of (92g) of HNO3 if reacted with (24g) of LIOH according
to the equation:
HNO3 + LIOH → LİNO3 + H2 O
wwww m
a) 46
b) 29
c) 2
d) 11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax