Limiting reactants Haley Aqueous sulfuric acid (H,SO) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium sulfate (Na, So,) and liquid water (H,O). Suppose 7.8 g of sulfuric acid is mixed with 3.97 g of sodium hydroxide. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.
Limiting reactants Haley Aqueous sulfuric acid (H,SO) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium sulfate (Na, So,) and liquid water (H,O). Suppose 7.8 g of sulfuric acid is mixed with 3.97 g of sodium hydroxide. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter1: The Nature Of Chemistry
Section: Chapter Questions
Problem 120QRT
Related questions
Question
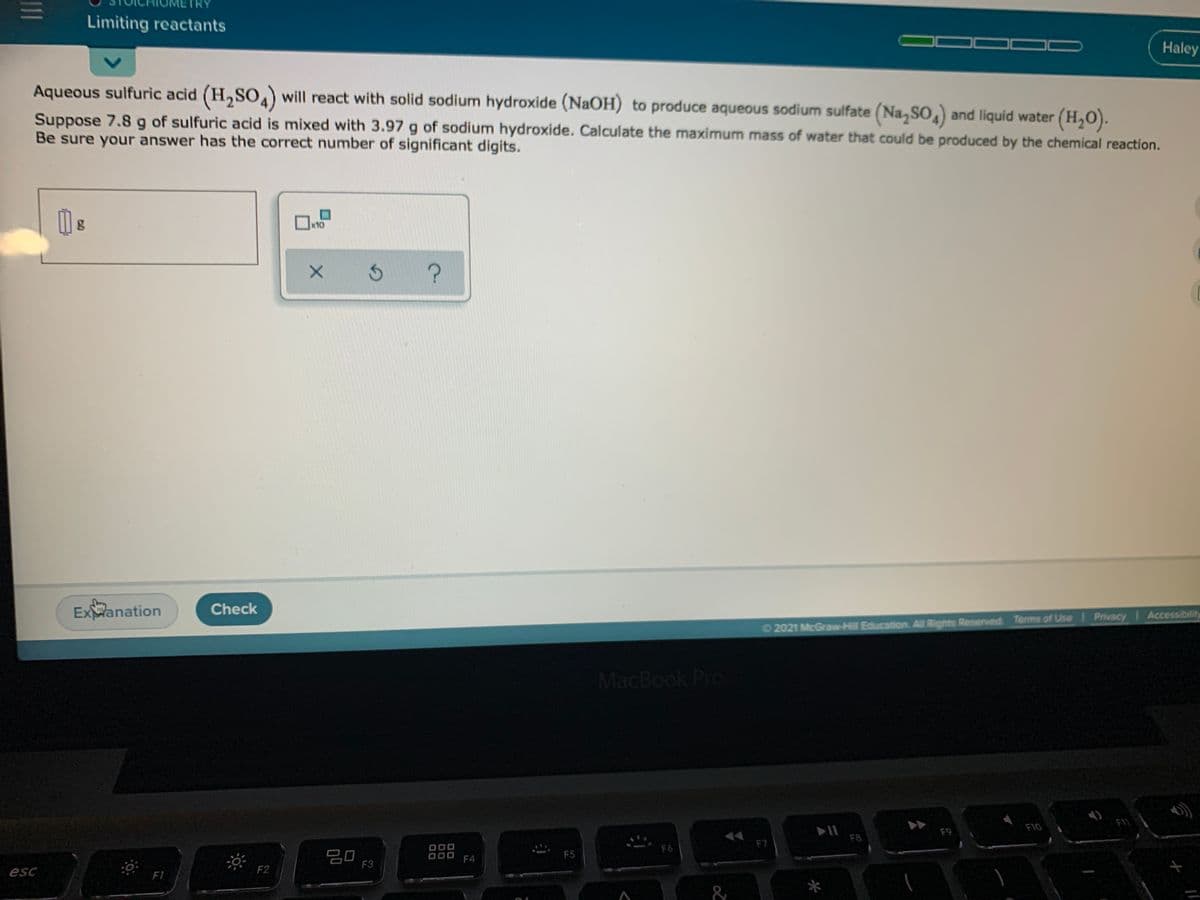
Transcribed Image Text:Limiting reactants
Haley
Aqueous sulfuric acid (H,SO) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium sulfate (Na,SO, and liquid water (H,0).
Suppose 7.8 g of sulfuric acid is mixed with 3.97 g of sodium hydroxide. Calculate the maximum mass of water that could be produced by the chemical reaction.
Be sure your answer has the correct number of significant digits.
x10
ExWanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved Terms of Use Privacy Accessibility
MacBook Pro
F11
F10
F9
F8
F7
O00
F6
20
F3
O00
F4
F5
esc
F1
F2
11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning