LizS2O3 + Br2 → LİBR + LİS4O6 b) Write the products for each of the following reactions (remember to add phase labels for each compound): i) Ca(OH)2(aq) + Na2CO3(aq) + A + B ii) C4H10(9) + O2(g) → C + D c) Write the nett ionic equation for the equation given below (show all steps): AGNO3(aq) + NH4|(aq) → Agle) + NH4NO3(aq)
LizS2O3 + Br2 → LİBR + LİS4O6 b) Write the products for each of the following reactions (remember to add phase labels for each compound): i) Ca(OH)2(aq) + Na2CO3(aq) + A + B ii) C4H10(9) + O2(g) → C + D c) Write the nett ionic equation for the equation given below (show all steps): AGNO3(aq) + NH4|(aq) → Agle) + NH4NO3(aq)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 81GQ: Balance equations for these reactions that occur in aqueous solution, and then classify each as a...
Related questions
Question
100%
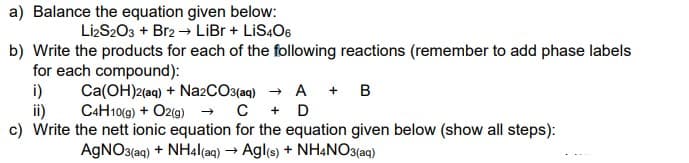
Transcribed Image Text:a) Balance the equation given below:
LizS203 + Br2 → LiBr + LIS4O6
b) Write the products for each of the following reactions (remember to add phase labels
for each compound):
i)
Ca(OH)2(aq) + Na2CO3(aq) → A + B
ii)
C4H10(9) + O2(g) → C + D
c) Write the nett ionic equation for the equation given below (show all steps):
AGNO3(aq) + NH4|(aq) → Agl(s) + NH4NO3(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning