Gravimetric analysis of a mixture gives the percentage of a pure substance (either element or compound) present in the mixture. Sulfates are widely distributed in nature in natural water (which has a density of 1.029 g/mL) in concentrations ranging from a few to several thousand milligrams per litre. Sulfates are important in handling and treatment of wastewater. Below is the procedure for a precipitation gravimetric analysis. Experiment 250 mL of the unknown natural water sample was measured into a 500 mL beaker and then 3 mL 1 M HCl was added. Observation A colourless solution formed. The solution became cloudy. The solution was heated to near boiling point and an amount of 80 mL 0.5 M BaCI, was slowly added to it with continuous stirring. The precipitate was allowed to sediment for about 30 minutes. There was cloudiness when two drops of BaCl, were added to Two drops of BaCl, were added and if cloudiness was again observed, 40 mL BaCI, was added to the solution and allowed to sediment. This test was performed continuously until no cloudiness formed. the solution. The recorded mass was 1.058 g. A filter paper (of constant weight) was cooled in a desiccator, weighed, and its mass noted. The precipitate is BasO,. The solution was filtered and cooled in a desiccator, weighed, and its mass noted. The filtrate and filter paper had a recorded mass of 3.5363g. Calculate the percentage composition of sulfate in the natural water by mass.
Gravimetric analysis of a mixture gives the percentage of a pure substance (either element or compound) present in the mixture. Sulfates are widely distributed in nature in natural water (which has a density of 1.029 g/mL) in concentrations ranging from a few to several thousand milligrams per litre. Sulfates are important in handling and treatment of wastewater. Below is the procedure for a precipitation gravimetric analysis. Experiment 250 mL of the unknown natural water sample was measured into a 500 mL beaker and then 3 mL 1 M HCl was added. Observation A colourless solution formed. The solution became cloudy. The solution was heated to near boiling point and an amount of 80 mL 0.5 M BaCI, was slowly added to it with continuous stirring. The precipitate was allowed to sediment for about 30 minutes. There was cloudiness when two drops of BaCl, were added to Two drops of BaCl, were added and if cloudiness was again observed, 40 mL BaCI, was added to the solution and allowed to sediment. This test was performed continuously until no cloudiness formed. the solution. The recorded mass was 1.058 g. A filter paper (of constant weight) was cooled in a desiccator, weighed, and its mass noted. The precipitate is BasO,. The solution was filtered and cooled in a desiccator, weighed, and its mass noted. The filtrate and filter paper had a recorded mass of 3.5363g. Calculate the percentage composition of sulfate in the natural water by mass.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 106AE: Many over-the-counter antacid tablets are now formulated using calcium carbonate as die active...
Related questions
Question
Gravimetric Analysis.
Need aid.
Thanks
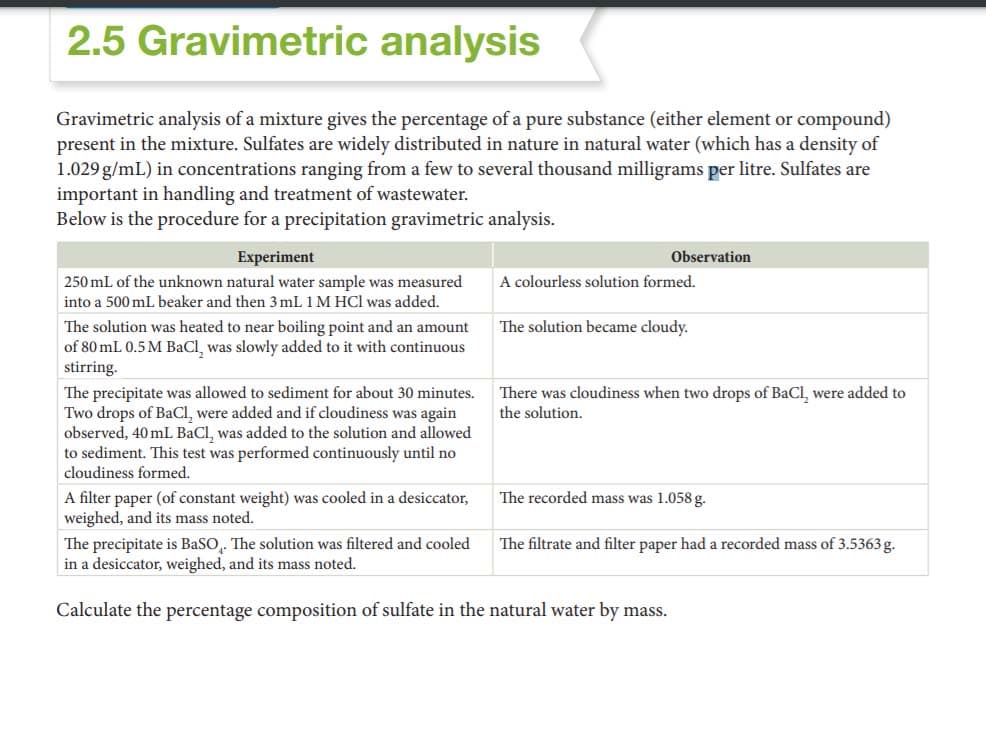
Transcribed Image Text:2.5 Gravimetric analysis
Gravimetric analysis of a mixture gives the percentage of a pure substance (either element or compound)
present in the mixture. Sulfates are widely distributed in nature in natural water (which has a density of
1.029 g/mL) in concentrations ranging from a few to several thousand milligrams per litre. Sulfates are
important in handling and treatment of wastewater.
Below is the procedure for a precipitation gravimetric analysis.
Experiment
Observation
250 mL of the unknown natural water sample was measured
A colourless solution formed.
into a 500 mL beaker and then 3 mL 1 M HCl was added.
The solution was heated to near boiling point and an amount
of 80 mL 0.5 M BaCl, was slowly added to it with continuous
stirring.
The solution became cloudy.
The precipitate was allowed to sediment for about 30 minutes.
Two drops of BaCl, were added and if cloudiness was again
observed, 40 mL BaCl, was added to the solution and allowed
to sediment. This test was performed continuously until no
cloudiness formed.
There was cloudiness when two drops of BaCl, were added to
the solution.
A filter paper (of constant weight) was cooled in a desiccator,
weighed, and its mass noted.
The recorded mass was 1.058g.
The precipitate is BaSO,. The solution was filtered and cooled
in a desiccator, weighed, and its mass noted.
The filtrate and filter paper had a recorded mass of 3.5363g.
Calculate the percentage composition of sulfate in the natural water by mass.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning