MAKE A METHODOLOGY : MATERIALS/ REAGENTS WITH SCHEMATIC DIAGRAM . THE PICTURE UPLOADED SERVES AS A GUIDE, DO THE SAME THING BASED FROM THE PIC. Procedure: Place 20 drops of each of 0.2M CuSO4 in a centrifuge tube. Then add 5 drops of freshly prepared 6M (NH4)2S to each centrifuge tube. Record your observation. Centrifuge the mixtures for 3 mins. and decant the supernatant liquid. Add 10 drops of DI water and 10 drops of 6M HNO3. Stir and boil the mixture in a hot water bath for 5 mins. centrifuge the mixture for 3 mins. Decant the supernatant solution from procedure 3. Discard the To each of the supernatant liquid, add 6 drops of 6M H2SO4. Allow themixtures to cool and 5 drops of DI water. To the mixtures in procedure 4, add few drops of concentrated NH3 until the solution becomes basic. Confirm using a litmus paper. Record your observation. Add 1 drop of 5M NaOH and 1 drop of 0.1 M SnCl2 to the centrifuge tube containing Bi3+ ion , centrifuge and decant the supernatant liquid. To the solution containing Sn 2+ion , add 6M HCl , dropwise in the test tube. Stir well. Add excess HCl in the solution. Centrifuge the mixture and record observation. To the solutions containing Sb+3, add few drops of H2s and few drops of ammonium polyphosphate in the solution. Record your observation.
MAKE A METHODOLOGY : MATERIALS/ REAGENTS WITH SCHEMATIC DIAGRAM . THE PICTURE UPLOADED SERVES AS A GUIDE, DO THE SAME THING BASED FROM THE PIC. Procedure: Place 20 drops of each of 0.2M CuSO4 in a centrifuge tube. Then add 5 drops of freshly prepared 6M (NH4)2S to each centrifuge tube. Record your observation. Centrifuge the mixtures for 3 mins. and decant the supernatant liquid. Add 10 drops of DI water and 10 drops of 6M HNO3. Stir and boil the mixture in a hot water bath for 5 mins. centrifuge the mixture for 3 mins. Decant the supernatant solution from procedure 3. Discard the To each of the supernatant liquid, add 6 drops of 6M H2SO4. Allow themixtures to cool and 5 drops of DI water. To the mixtures in procedure 4, add few drops of concentrated NH3 until the solution becomes basic. Confirm using a litmus paper. Record your observation. Add 1 drop of 5M NaOH and 1 drop of 0.1 M SnCl2 to the centrifuge tube containing Bi3+ ion , centrifuge and decant the supernatant liquid. To the solution containing Sn 2+ion , add 6M HCl , dropwise in the test tube. Stir well. Add excess HCl in the solution. Centrifuge the mixture and record observation. To the solutions containing Sb+3, add few drops of H2s and few drops of ammonium polyphosphate in the solution. Record your observation.
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 5P
Related questions
Question
MAKE A METHODOLOGY : MATERIALS/ REAGENTS WITH SCHEMATIC DIAGRAM .
THE PICTURE UPLOADED SERVES AS A GUIDE, DO THE SAME THING BASED FROM THE PIC.
Procedure:
- Place 20 drops of each of 0.2M CuSO4 in a centrifuge tube. Then add 5 drops of freshly prepared 6M (NH4)2S to each centrifuge tube. Record your observation.
- Centrifuge the mixtures for 3 mins. and decant the supernatant liquid. Add 10 drops of DI water and 10 drops of 6M HNO3. Stir and boil the mixture in a hot water bath for 5 mins.
- centrifuge the mixture for 3 mins.
- Decant the supernatant solution from procedure 3. Discard the To each of the supernatant liquid, add 6 drops of 6M H2SO4. Allow themixtures to cool and 5 drops of DI water.
- To the mixtures in procedure 4, add few drops of concentrated NH3 until the solution becomes basic. Confirm using a litmus paper. Record your observation.
- Add 1 drop of 5M NaOH and 1 drop of 0.1 M SnCl2 to the centrifuge tube containing Bi3+ ion , centrifuge and decant the supernatant liquid.
- To the solution containing Sn 2+ion , add 6M HCl , dropwise in the test tube. Stir well. Add excess HCl in the solution.
- Centrifuge the mixture and record observation.
- To the solutions containing Sb+3, add few drops of H2s and few drops of ammonium polyphosphate in the solution. Record your observation.
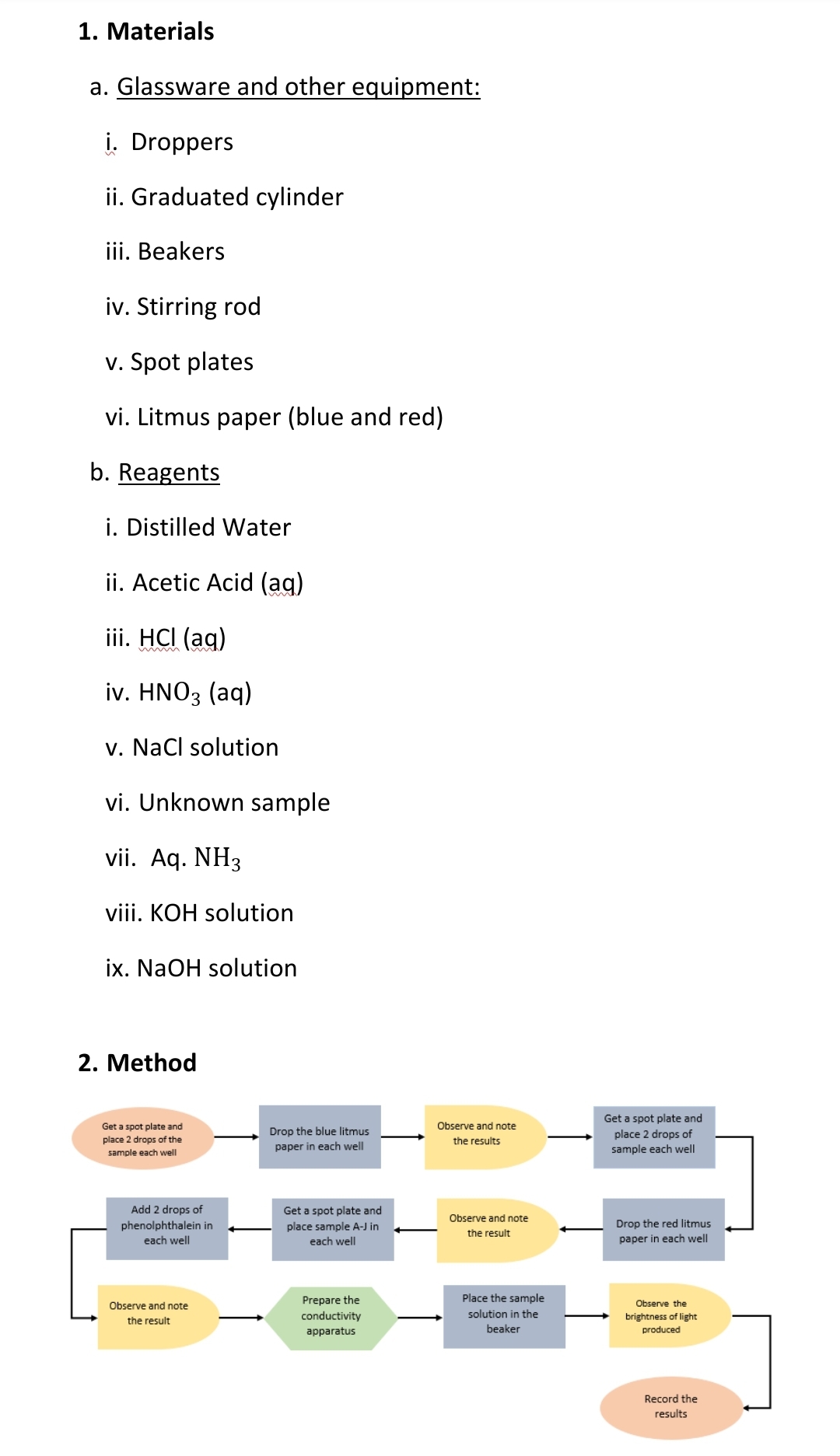
Transcribed Image Text:1. Materials
a. Glassware and other equipment:
i. Droppers
ii. Graduated cylinder
iii. Beakers
iv. Stirring rod
v. Spot plates
vi. Litmus paper (blue and red)
b. Reagents
i. Distilled Water
ii. Acetic Acid (ag)
iii. HCI (ag)
iv. ΗNO3 (aq)
v. Nacl solution
vi. Unknown sample
vii. Aq. NH3
viii. KOH solution
ix. NaOH solution
2. Method
Get a spot plate and
Get a spot plate and
Observe and note
Drop the blue litmus
place 2 drops of
place 2 drops of the
the results
paper in each well
sample each well
sample each well
Add 2 drops of
phenolphthalein in
each well
Get a spot plate and
Observe and note
place sample A-J in
each well
Drop the red litmus
paper in each well
the result
Place the sample
Prepare the
conductivity
Observe and note
Observe the
solution in the
brightness of light
produced
resu
apparatus
beaker
Record the
results
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning