Match the left phrase with the right phrase. Choose the best match. Non-crystalline Cubic structure Diamond CHOOSE: • Amorphous • 4 atoms/unit cell • Two angles equal; no HCP structure Many grains Miller-Bravais system Crystal defect sides Four-axes coordinate system Imperfection 6 atoms/unit cell crystalline Ordered 3D arrays Polycrystals All angles equal; all sides equal • All angles equal; 2 sides equal Single crystal Intermediate density values
Match the left phrase with the right phrase. Choose the best match. Non-crystalline Cubic structure Diamond CHOOSE: • Amorphous • 4 atoms/unit cell • Two angles equal; no HCP structure Many grains Miller-Bravais system Crystal defect sides Four-axes coordinate system Imperfection 6 atoms/unit cell crystalline Ordered 3D arrays Polycrystals All angles equal; all sides equal • All angles equal; 2 sides equal Single crystal Intermediate density values
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter21: The Solid State: Crystals
Section: Chapter Questions
Problem 21.7E: How many different unit cells can a crystal have if the unit cell a has all 90 angles between its...
Related questions
Question
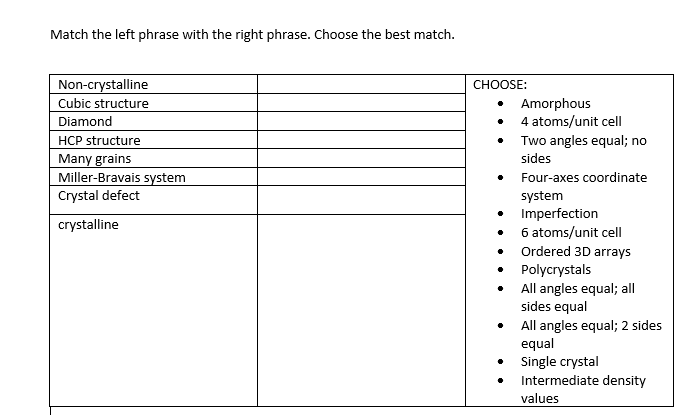
Transcribed Image Text:Match the left phrase with the right phrase. Choose the best match.
Non-crystalline
Cubic structure
Diamond
CHOOSE:
• Amorphous
• 4 atoms/unit cell
• Two angles equal; no
HCP structure
Many grains
Miller-Bravais system
Crystal defect
sides
Four-axes coordinate
system
Imperfection
6 atoms/unit cell
crystalline
Ordered 3D arrays
Polycrystals
All angles equal; all
sides equal
• All angles equal; 2 sides
equal
Single crystal
Intermediate density
values
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning