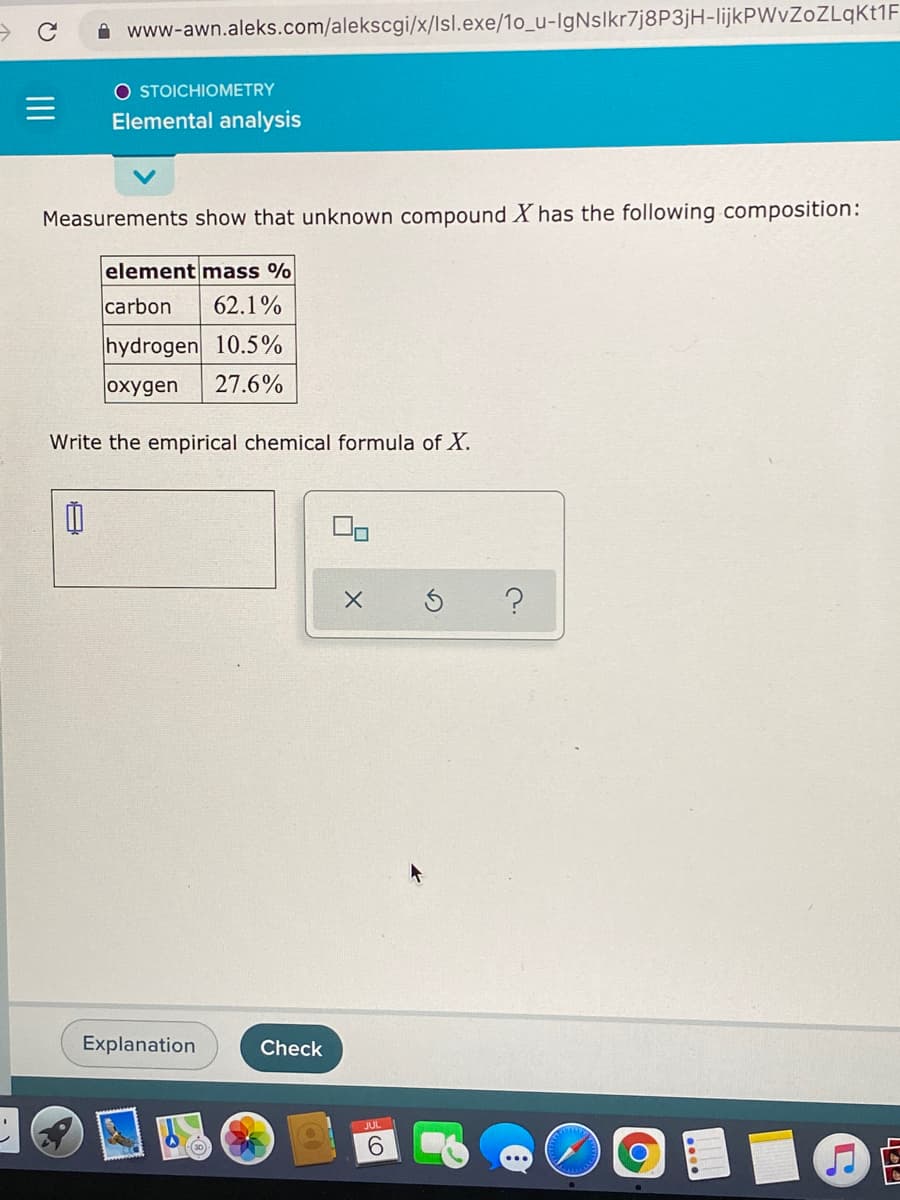
Measurements show that unknown compound X has the following composition: element mass % carbon 62.1% hydrogen 10.5% oxygen 27.6% Write the empirical chemical formula of X.
Measurements show that unknown compound X has the following composition: element mass % carbon 62.1% hydrogen 10.5% oxygen 27.6% Write the empirical chemical formula of X.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 5RQ: Fig. 5-5 illustrates a schematic diagram of a combustion device used to analyze organic compounds....
Related questions
Question
Transcribed Image Text:A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZoZLqKt1F
O STOICHIOMETRY
Elemental analysis
Measurements show that unknown compound X has the following composition:
element mass %
carbon
62.1%
hydrogen 10.5%
oxygen
27.6%
Write the empirical chemical formula of X.
Explanation
Check
JUL
6.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning