Mercury ions, Hg(1) and Hg(II), are poisonous due to their ability to displace other metal ions in enzymes and disrupt the biological function. a) Are these ions hard or soft? b) Common ligands for metal ions in biological environments are amino acids (building blocks of proteins). Of the amino acids depicted below (focus on the highlighted atoms), which would be the best ligand for Hg(1) and Hg(II), and why? H H O H3Nt ÇH2 H3N--c CH2 H H3Nt H3N--c CH2 H3N*. H3Nt CH2 CH CH2 CH2 OH но CH3 SH он H2Ñ H2N Serine (S) Ser Threonine (T) Cysteine (C) Cys Tyrosine (Y) Asparagine (N) Glutamine (Q) Asn Thr Тyr Gln c) Other metal ions that are toxic at certain concentrations include cadmium, chromium, iron, arsenic, and lead. For each of these metals, predict whether they would have similar chemistry to mercury using HSAB concepts.- (Note that toxicity is dose-dependent; even water can be poisonous at too high a concentration in the body)
Mercury ions, Hg(1) and Hg(II), are poisonous due to their ability to displace other metal ions in enzymes and disrupt the biological function. a) Are these ions hard or soft? b) Common ligands for metal ions in biological environments are amino acids (building blocks of proteins). Of the amino acids depicted below (focus on the highlighted atoms), which would be the best ligand for Hg(1) and Hg(II), and why? H H O H3Nt ÇH2 H3N--c CH2 H H3Nt H3N--c CH2 H3N*. H3Nt CH2 CH CH2 CH2 OH но CH3 SH он H2Ñ H2N Serine (S) Ser Threonine (T) Cysteine (C) Cys Tyrosine (Y) Asparagine (N) Glutamine (Q) Asn Thr Тyr Gln c) Other metal ions that are toxic at certain concentrations include cadmium, chromium, iron, arsenic, and lead. For each of these metals, predict whether they would have similar chemistry to mercury using HSAB concepts.- (Note that toxicity is dose-dependent; even water can be poisonous at too high a concentration in the body)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter22: The Chemistry Of The Transistion Elements
Section: Chapter Questions
Problem 78GQ: The glycinate ion, H2NCH2CO2, formed by deprotonation of the amino acid glycine, can function as a...
Related questions
Question
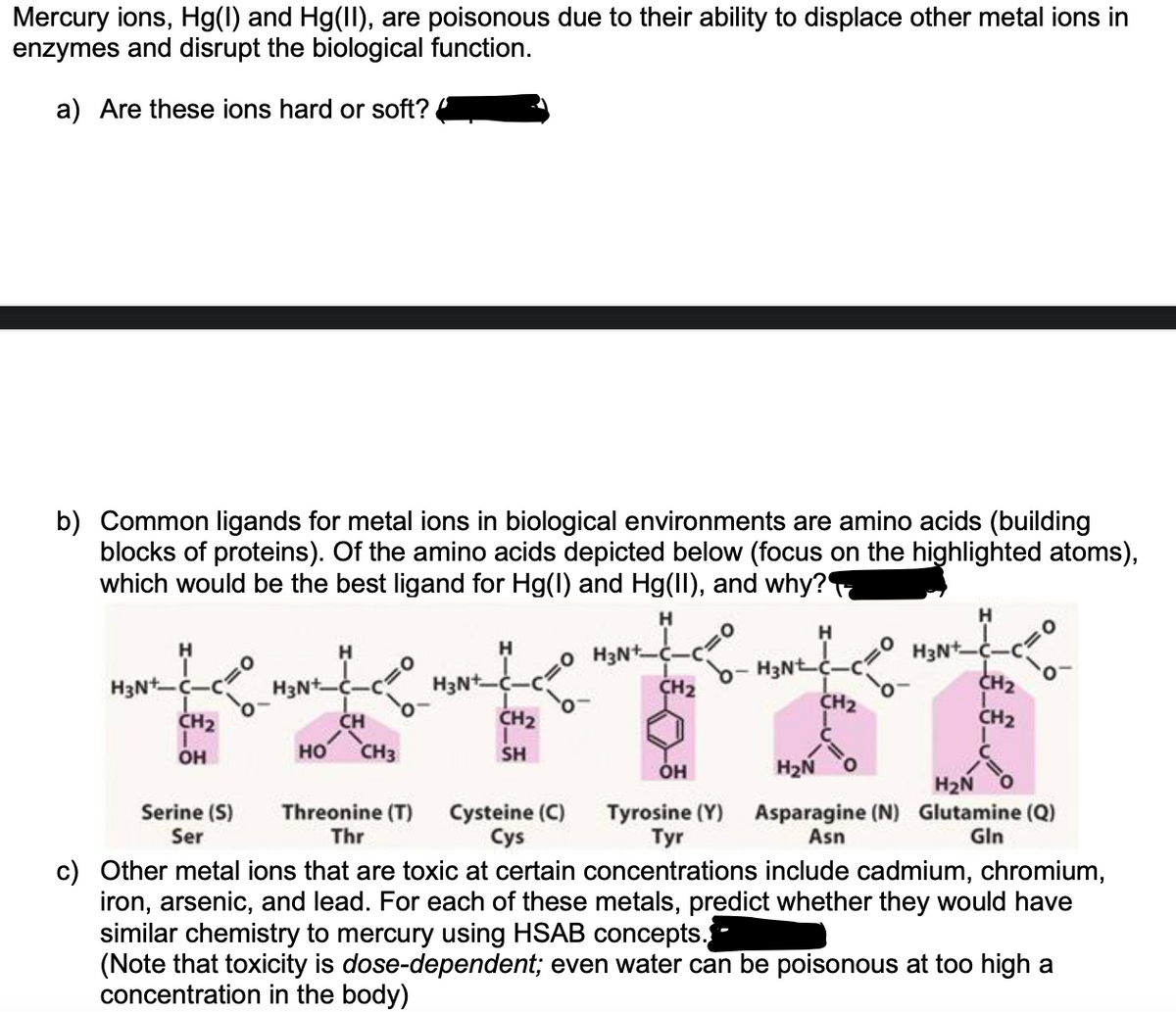
Transcribed Image Text:Mercury ions, Hg(1) and Hg(II), are poisonous due to their ability to displace other metal ions in
enzymes and disrupt the biological function.
a) Are these ions hard or soft?
b) Common ligands for metal ions in biological environments are amino acids (building
blocks of proteins). Of the amino acids depicted below (focus on the highlighted atoms),
which would be the best ligand for Hg(1) and Hg(II), and why?
H
H
H
O H3Nt
CH2
H
H
H3N* -c
CH2
H3Nt C-c
CH2
H3Nt
CH2
H3N+-C-c
CH
CH2
HO CH3
OH
SH
он
H2N
H2N
Serine (S)
Ser
Threonine (T)
Thr
Cysteine
Сys
Tyrosine (Y) Asparagine (N) Glutamine (Q)
Asn
Тyr
Gln
c) Other metal ions that are toxic at certain concentrations include cadmium, chromium,
iron, arsenic, and lead. For each of these metals, predict whether they would have
similar chemistry to mercury using HSAB concepts.
(Note that toxicity is dose-dependent; even water can be poisonous at too high a
concentration in the body)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning