Mercury(II) sulfide (HgS) exists in two different crystalline forms. In cinnabar, the band gap is 3.2 × 10¬19 J; in meta- cinnabar, it is 2.6 × 10-19 J. In some old paintings with improperly formulated paints, the pigment vermilion (cin- nabar) has transformed to metacinnabar on exposure to light. Describe the color change that results.
Mercury(II) sulfide (HgS) exists in two different crystalline forms. In cinnabar, the band gap is 3.2 × 10¬19 J; in meta- cinnabar, it is 2.6 × 10-19 J. In some old paintings with improperly formulated paints, the pigment vermilion (cin- nabar) has transformed to metacinnabar on exposure to light. Describe the color change that results.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter22: Inorganic Materials
Section: Chapter Questions
Problem 36P
Related questions
Question
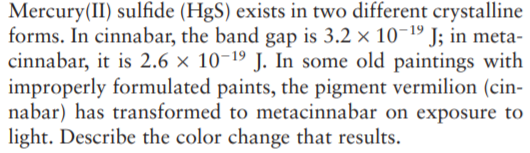
Transcribed Image Text:Mercury(II) sulfide (HgS) exists in two different crystalline
forms. In cinnabar, the band gap is 3.2 × 10¬19 J; in meta-
cinnabar, it is 2.6 × 10-19 J. In some old paintings with
improperly formulated paints, the pigment vermilion (cin-
nabar) has transformed to metacinnabar on exposure to
light. Describe the color change that results.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning