Metallic strontium crystallizes in a face-centered cubic lattice. The volume of the unit cell is 2.25 × 10° pm3. What is the density. of strontium metal in g/cm3?
Metallic strontium crystallizes in a face-centered cubic lattice. The volume of the unit cell is 2.25 × 10° pm3. What is the density. of strontium metal in g/cm3?
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter8: Electrochemistry And Ionic Solutions
Section: Chapter Questions
Problem 8.75E: Under what conditions does the extended Debye-Huckel law, equation 8.52, become the Debye-Hckel...
Related questions
Question
Transcribed Image Text:nrome
File
Edit
View
History
Bookmarks Profiles
Tab
Window Help
O Ap
Aol. AC
Aol. A No
Er i Lc G th GmN N
G m sf caGn
Aol. ac
St
St
A Li
New 1
->
A app.101edu.co
pps Gmail
O YouTube
W Maps
F Login - CAS - Cen.
BB Blackboard Learn G Second Request -.
101 Chem101: Transfo...
Question 30 of 33
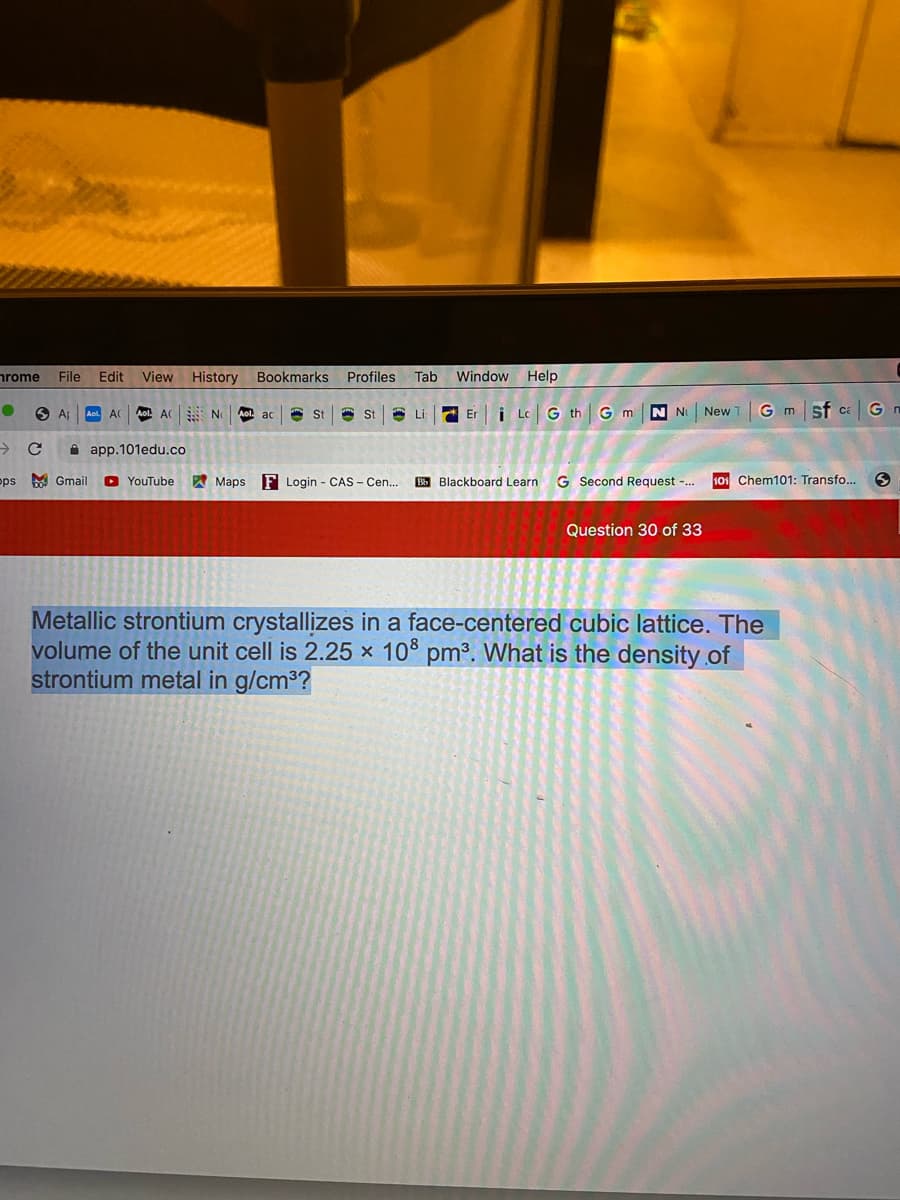
Metallic strontium crystallizes in a face-centered cubic lattice. The
volume of the unit cell is 2.25 x 10° pm³. What is the density.of
strontium metal in g/cm3?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,