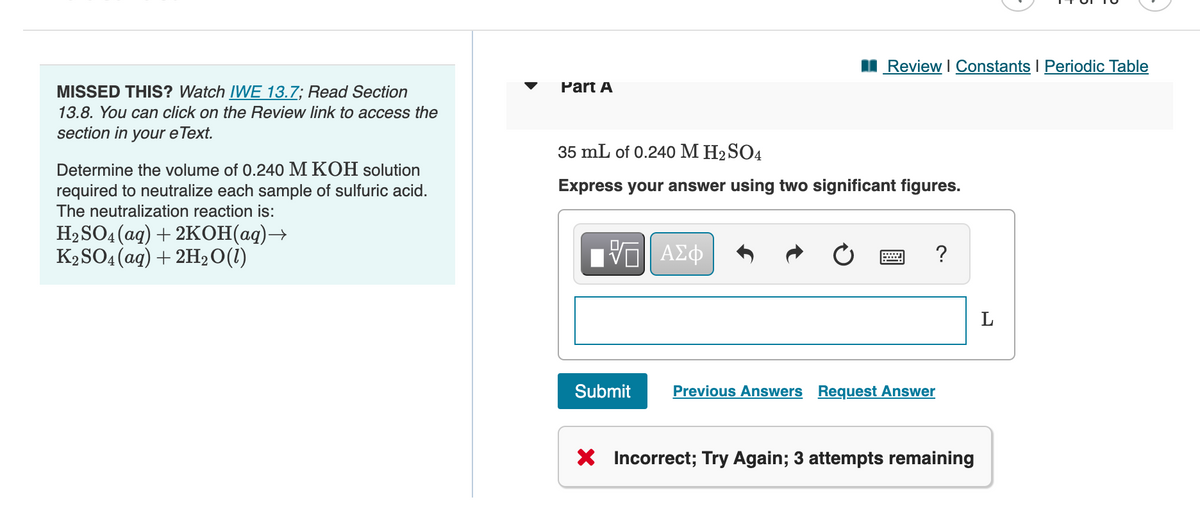
MISSED THIS? Watch IWE 13.7; Read Section 13.8. You can click on the Review link to access the Part A section in your eText. 35 mL of 0.240 M H2SO4 Determine the volume of 0.240 M KOH solution Express your answer using two significant figures. required to neutralize each sample of sulfuric acid. The neutralization reaction is: HaSOA (ag) + 2КОН(ад) — K2SO4 (aq) + 2H20(1) ?
MISSED THIS? Watch IWE 13.7; Read Section 13.8. You can click on the Review link to access the Part A section in your eText. 35 mL of 0.240 M H2SO4 Determine the volume of 0.240 M KOH solution Express your answer using two significant figures. required to neutralize each sample of sulfuric acid. The neutralization reaction is: HaSOA (ag) + 2КОН(ад) — K2SO4 (aq) + 2H20(1) ?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 2CR
Related questions
Question
100%
Part A
35 mLmL of 0.240 MM H2SO4H2SO4
Express your answer using two significant figures.
.............. L ?
Part B
165 mLmL of 0.115 MM H2SO4H2SO4
Express your answer using three significant figures.
.............L?
Part C
70 mLmL of 0.115 MM H2SO4H2SO4
Express your answer using two significant figures.
................L?
Transcribed Image Text:Review I Constants I Periodic Table
MISSED THIS? Watch IWE 13.7; Read Section
Part A
13.8. You can click on the Review link to access the
section in your e Text.
35 mL of 0.240 M H2 SO4
Determine the volume of 0.240 M KOH solution
required to neutralize each sample of sulfuric acid.
The neutralization reaction is:
Express your answer using two significant figures.
H2 SO4 (ag) + 2KOH(aq)→
K2SO4 (aq) + 2H2O(1)
ΑΣφ
?
L
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 3 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning