Various ways to measure and represent concentration are available. The following lists the common concentration methods discussed in Chemistry 20. Match the concentration method with the correct description. A way of expressing very dilute concentrations of a substance Choose... Choose... A way of expressing extremely dilute concentrations of a substance Used for solutes that are solids in pure form and are dissolved in a liquid solvent Choose... Used for solutions prepared by dissolving a liquid solute in a liquid solvent amount concentration (mol/L) percentage by volume (% V/V) percentage by mass (% W/W) percentage mass by volume (% W/V) parts per billion (ppb) Used when both the solute and solvent are measured in grams parts per million (ppm) Used when the solute is measured as a molar amount in one litre of solution amount concentration (mol/L)
Various ways to measure and represent concentration are available. The following lists the common concentration methods discussed in Chemistry 20. Match the concentration method with the correct description. A way of expressing very dilute concentrations of a substance Choose... Choose... A way of expressing extremely dilute concentrations of a substance Used for solutes that are solids in pure form and are dissolved in a liquid solvent Choose... Used for solutions prepared by dissolving a liquid solute in a liquid solvent amount concentration (mol/L) percentage by volume (% V/V) percentage by mass (% W/W) percentage mass by volume (% W/V) parts per billion (ppb) Used when both the solute and solvent are measured in grams parts per million (ppm) Used when the solute is measured as a molar amount in one litre of solution amount concentration (mol/L)
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter6: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 123CP: The units of parts per million (ppm) and parts per billion (ppb) are commonly used by environmental...
Related questions
Question
U guys gotta help me on this
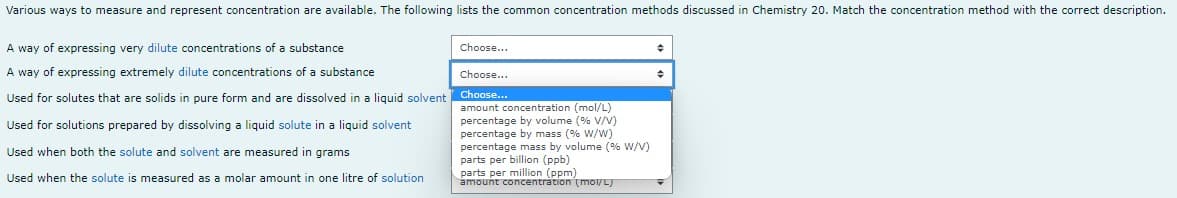
Transcribed Image Text:Various ways to measure and represent concentration are available. The following lists the common concentration methods discussed in Chemistry 20. Match the concentration method with the correct description.
A way of expressing very dilute concentrations of a substance
Choose...
Choose...
A way of expressing extremely dilute concentrations of a substance
Choose...
Used for solutes that are solids in pure form and are dissolved in a liquid solvent
Used for solutions prepared by dissolving a liquid solute in a liquid solvent
amount concentration (mol/L)
percentage by volume (% V/V)
percentage by mass (% W/W)
percentage mass by volume (% W/V)
parts per billion (ppb)
Used when both the solute and solvent are measured in grams
parts per million (ppm)
Used when the solute is measured as a molar amount in one litre of solution
amount concentration (mol/L)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning